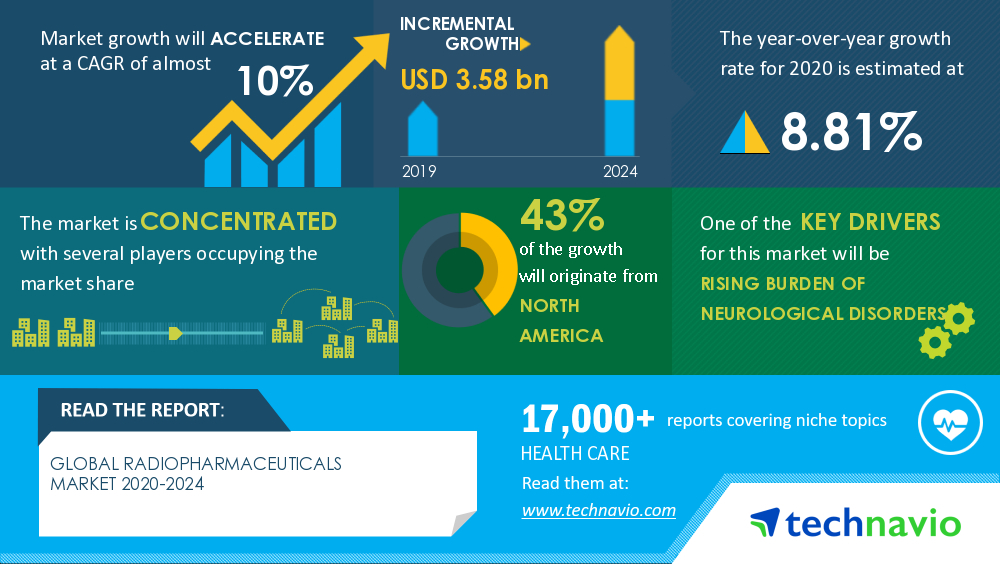
Nuclear medicine has transitioned from a niche field to a groundbreaking modality for cancer diagnosis and therapy. The seismic shift is spearheaded by pioneers like Advanced Innovative Partners (AIP), whose visionary approach aims to maximize the clinical impact of nuclear medicine. As the global radiopharmaceutical market burgeons to a staggering $5.2 billion in 2022, it is anticipated to achieve a value of USD 9.75 Billion by the year 2030, exhibiting a Compound Annual Growth Rate (CAGR) of 9.4% during the period spanning from 2023 to 2030. This transformative journey is expected to reshape the entire landscape of cancer care.
Precision in Action: The Radiopharmaceutical Renaissance
At the heart of nuclear medicine’s ascent is the precision it offers through radiopharmaceuticals that selectively target tumors. This nuanced approach, driven by isotopes like lutetium-177, actinium-225, copper-64, and gallium-68, ushers in a new era of personalized and theranostic cancer care. AIP’s focus on pairing imaging agents with molecularly targeted treatments showcases the power of synergy in this evolving field.
Co-Founder, CEO & President Roseanne “Rose” Satz, a visionary driving force behind AIP, articulates the profound impact of nuclear medicine: “Nuclear medicine enables both detection and treatment of tumors using trace amounts of radioactive material. This dual capability heralds a paradigm shift, offering not only early detection but also treatment with minimal impact on healthy tissues.”
Beyond Chemotherapy: The Rise of Targeted Radionuclide Therapy
For cancer patients, the prospect of targeted radionuclide therapy is a beacon of hope, promising efficacious treatment with fewer debilitating side effects compared to traditional chemotherapy and radiation. The FDA’s approval of several radiopharmaceutical cancer therapies underscores the rapid evolution and growing acceptance of this transformative approach.
“We aim to maximize these treatments’ impact through our integrated capabilities spanning discovery, clinical trials, manufacturing, and commercialization,” states Satz. AIP’s commitment to a comprehensive approach positions them as a driving force in advancing nuclear medicine from theoretical promise to practical, life-changing applications.
You can access the current roster of all FDA-approved radiopharmaceuticals in a portable document format (PDF) through this link. According to the United States Pharmacopeia (USP) <825>, conventionally manufactured drug products (e.g., NDA, ANDA) are mandated for Immediate Use. Nuclear medicine practitioners utilizing radiopharmaceuticals from sources not listed in these tables might be employing unauthorized copies.
Unraveling the Dynamics of Radiopharmaceutical Markets
The market dynamics of radiopharmaceuticals are intricately categorized based on key factors such as radioisotope, application, type, end user, and region. In the realm of radioisotopes, the market delineates itself into distinct segments, including Technetium-99m, Fluorine-18, Iodine I, Gallium-68, and various other variants. In the domain of application, the market exhibits a nuanced classification, with segments devoted to cancer, cardiology, and other medical applications. Furthermore, the market type undergoes scrutiny, branching into diagnostic and therapeutic categories. The end-user spectrum encompasses hospitals and clinics, medical imaging centers, as well as other entities like diagnostic centers and research institutes. The geographical footprint of the market extends across North America, Europe, Asia-Pacific, and LAMEA.
Charting AIP’s Trailblazing Journey in Nuclear Medicine
With an unwavering commitment spanning discovery, clinical trials, manufacturing, and commercialization, AIP assumes a pioneering role at the forefront of nuclear medicine’s clinical advancements. The trajectory of the company’s growth mirrors the burgeoning expansion of the field itself, offering not only innovative treatment options but also instilling a renewed sense of optimism among cancer patients.
As nuclear medicine ascends to a pivotal position in the battle against cancer, AIP, alongside other industry innovators, charts a transformative course. This course envisions a future where precision, efficacy, and personalized care seamlessly converge, pushing the boundaries of medical science and redefining the landscape of cancer care.
About Advanced Innovative Partners (AIP)
Advanced Innovative Partners (AIP) emerges as a prominent global clinical-stage biotechnology company, standing at the forefront of diagnostic and therapeutic advancements in the realm of targeted radiation. Founded in 2017, AIP boasts a skilled team with expertise spanning biotechnology, nuclear medicine, and molecular biology. Their commitment lies in the development of next-generation diagnostic and therapeutic radiopharmaceuticals, addressing critical needs in oncology, rare pediatric diseases, infectious diseases, and biomedical countermeasures.

At the heart of AIP’s operations is a robust pipeline, extending its reach to breast, lung, brain, and solid tumor cancers, as well as rare diseases. AIP navigates this expansive landscape with a reliable, secure global supply chain and a collaborative network, ensuring the seamless progression of their innovative products from development to delivery.
With lead programs advancing through Phase I/II/III clinical trials, AIP positions itself as a leader in the emerging market space of radiopharmaceutical diagnostics and therapeutics. The company’s success is underpinned by patented platform technologies that underscore their commitment to excellence in treatment and clinical management. AIP’s approach is product-centric, reflected in the creation of a pipeline comprising multiple drugs strategically targeting areas with significant market opportunities.
AIP’s mission revolves around expediting the discovery, development, and global availability of transformative therapies. Their focus extends beyond innovation, emphasizing the importance of rigorous clinical trials mandated by regulatory bodies. By navigating these regulatory pathways and obtaining approvals, AIP aims to make their investigational products accessible to patients worldwide.
Central to AIP’s identity is a dedication to pioneering targeted therapies and advancing disease detection and treatment. This commitment unfolds through a comprehensive, integrated approach encompassing proprietary tech platforms, strategic partnerships, and an unwavering patient-centric focus. AIP’s ultimate goal is to profoundly impact patient journeys by enabling accurate diagnoses at an earlier stage and providing treatments that are not only more powerful but also gentler, thereby offering patients the best chance for improved outcomes. In essence, AIP aspires to make a meaningful difference for individuals grappling with complex, aggressive, rare, and infectious diseases worldwide.

Learn more about AIP from their Official Website.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Immunology & Oncology
Forging a Hopeful Outlook on Cancer Drug Development
The core of oncotherapy is still about reducing human suffering and facing the many challenges of this disease together.
Immunology & Oncology
Patterns, Profiles, and Patient Outcomes: AI in Oncotherapeutics
AI presents a beacon of hope in revolutionizing cancer management across various stages.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.