As cancer continues its relentless impact globally, the quest for more effective and targeted treatment options intensifies. Among the promising innovations, Peptide Receptor Radionuclide Therapy (PRRT) emerges as a frontrunner, with Florida-based Advanced Innovative Partners (AIP) spearheading groundbreaking research and development in this domain.

PRRT capitalizes on the overexpression of specific peptide receptors on the surfaces of cancer cells. The process involves linking radioactive isotopes to synthetic peptide ligands, forming a complex that exhibits high affinity to these receptors. Upon binding, the radiopeptide complex undergoes receptor-mediated internalization, precisely directing radiation to the tumor while sparing healthy cells.
AIP’s Gallium-68, Lutetium-177, & Copper-64 Radioisotopes
AIP’s focus centers on the development of PRRT utilizing gallium-68, lutetium-177, and copper-64 radioisotopes. Their extensive pipeline includes radiolabeled peptides designed to target somatostatin, neurotensin, bombesin, and other receptors expressed in various cancers, such as neuroendocrine tumors, gliomas, breast cancer, and prostate cancer.
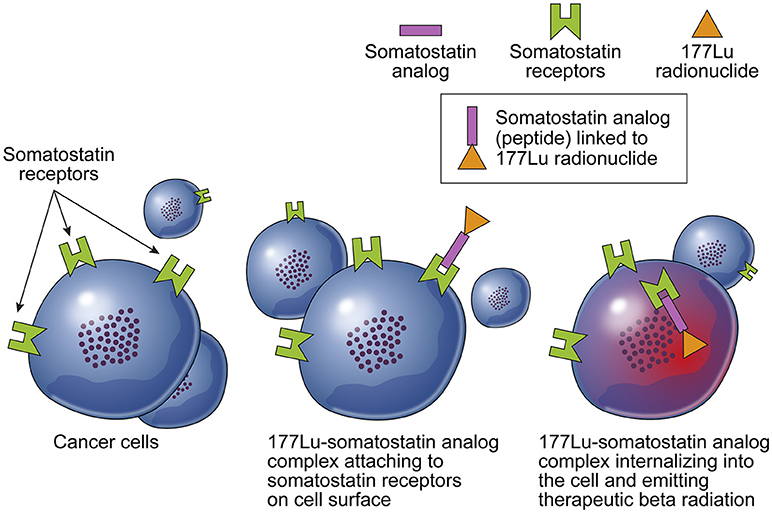
Dr. Stanley “Stan” Satz, Co-Founder, Chairman & Chief Scientific Officer of AIP, emphasizes the allure of PRRT, stating, “PRRT is intriguing because it offers a way to selectively deliver cytotoxic radiation payloads directly to malignant cells.” The optimization of pharmacokinetics and tumor targeting for each radiopeptide presents a unique opportunity to address specific cancer types in a personalized manner.
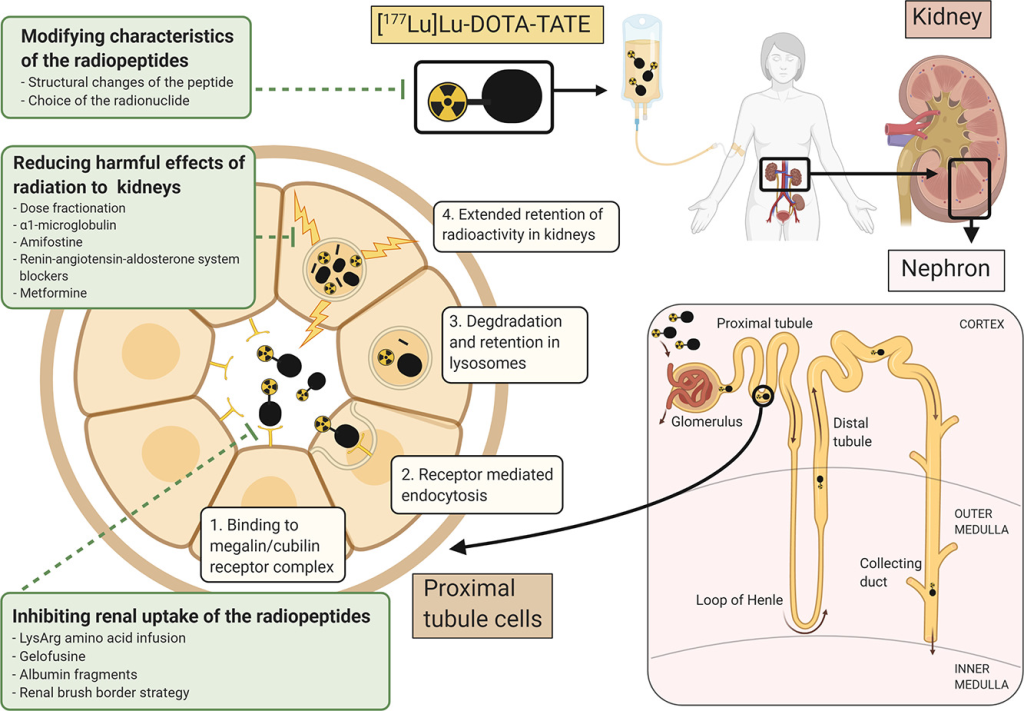
Theranostic Potential Unleashed: Companion Imaging Agents & Diagnostic Radioisotopes
Beyond its therapeutic impact, PRRT unveils theranostic potential. AIP pioneers the development of companion imaging agents, pairing peptides with diagnostic radioisotopes like gallium-68. This innovative approach allows for the identification of patients who stand to benefit from therapeutic radiopeptides before initiating the treatment.
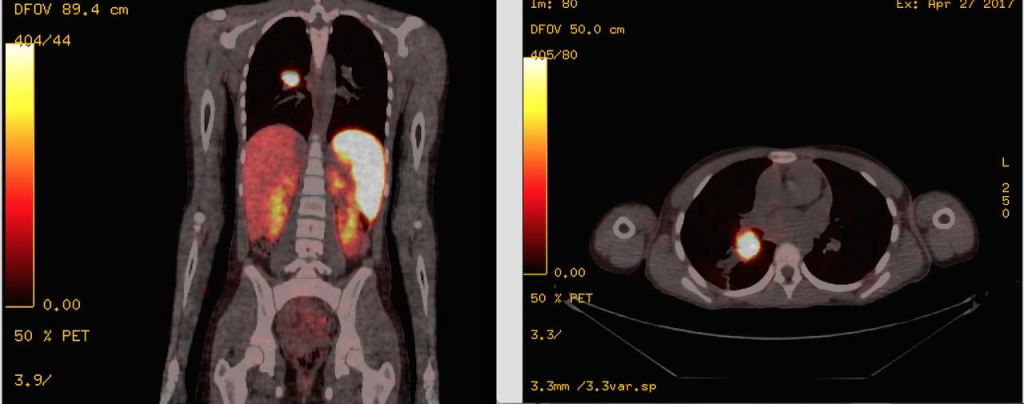
AIP’s strategy harnesses automated radiosynthesizers and cGMP facilities, ensuring the reliable production of clinical-grade radiopeptides. Their comprehensive approach, from discovery to clinical trials and eventual commercialization, underscores a commitment to providing cancer patients with targeted and effective PRRT options.

A Visionary Leap: Radiopeptides as the Future of Precision Oncotherapeutics
AIP Co-Founder, CEO & President Roseanne “Rose” Satz envisions radiopeptides as the next frontier in precision oncology drugs, stating, “Our goal is to improve cancer survival by progressing PRRT from promise to practice.” With a world-class team dedicated to advancing peptide receptor radionuclide therapies, AIP stands at the forefront of innovative cancer treatment. Their research not only signifies hope but also promises a paradigm shift for patients grappling with the most challenging malignancies. AIP’s commitment to advancing PRRT reinforces its position as a beacon of hope in the ongoing battle against cancer.
About Advanced Innovative Partners (AIP)
Advanced Innovative Partners (AIP) emerges as a prominent global clinical-stage biotechnology company, standing at the forefront of diagnostic and therapeutic advancements in the realm of targeted radiation. Founded in 2017, AIP boasts a skilled team with expertise spanning biotechnology, nuclear medicine, and molecular biology. Their commitment lies in the development of next-generation diagnostic and therapeutic radiopharmaceuticals, addressing critical needs in oncology, rare pediatric diseases, infectious diseases, and biomedical countermeasures.

At the heart of AIP’s operations is a robust pipeline, extending its reach to breast, lung, brain, and solid tumor cancers, as well as rare diseases. AIP navigates this expansive landscape with a reliable, secure global supply chain and a collaborative network, ensuring the seamless progression of their innovative products from development to delivery.
With lead programs advancing through Phase I/II/III clinical trials, AIP positions itself as a leader in the emerging market space of radiopharmaceutical diagnostics and therapeutics. The company’s success is underpinned by patented platform technologies that underscore their commitment to excellence in treatment and clinical management. AIP’s approach is product-centric, reflected in the creation of a pipeline comprising multiple drugs strategically targeting areas with significant market opportunities.
AIP’s mission revolves around expediting the discovery, development, and global availability of transformative therapies. Their focus extends beyond innovation, emphasizing the importance of rigorous clinical trials mandated by regulatory bodies. By navigating these regulatory pathways and obtaining approvals, AIP aims to make their investigational products accessible to patients worldwide.
Central to AIP’s identity is a dedication to pioneering targeted therapies and advancing disease detection and treatment. This commitment unfolds through a comprehensive, integrated approach encompassing proprietary tech platforms, strategic partnerships, and an unwavering patient-centric focus. AIP’s ultimate goal is to profoundly impact patient journeys by enabling accurate diagnoses at an earlier stage and providing treatments that are not only more powerful but also gentler, thereby offering patients the best chance for improved outcomes. In essence, AIP aspires to make a meaningful difference for individuals grappling with complex, aggressive, rare, and infectious diseases worldwide.

Learn more about AIP from their Official Website.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Immunology & Oncology
Forging a Hopeful Outlook on Cancer Drug Development
The core of oncotherapy is still about reducing human suffering and facing the many challenges of this disease together.
Immunology & Oncology
Patterns, Profiles, and Patient Outcomes: AI in Oncotherapeutics
AI presents a beacon of hope in revolutionizing cancer management across various stages.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.