Ethical considerations stand as a cornerstone in the pursuit of medical progress in guiding the delicate balance between scientific advancement and the rights of research subjects. At the heart of this discussion lies the involvement of minors in clinical research trials, a subject intricately woven with considerations of vulnerability, risk assessment, parental consent, and evolving notions of autonomy. Guided by the principles laid out in the World Medical Association’s Declaration of Helsinki, the ethical discourse surrounding minors’ participation in clinical trials is multi-faceted, reflecting a complex interplay of medical necessity, societal responsibility, and individual rights.
Navigating Vulnerability: The Ethical Imperatives
Central to the ethical discourse on minors’ participation in clinical trials is the recognition of their inherent vulnerability. Defined by incomplete physical and psychological development, minors occupy a unique position that necessitates special considerations in research endeavors. The imperative to protect them from harm looms large, underpinned by a fundamental commitment to safeguarding their rights and interests. Yet, as articulated by the Council for International Organizations of Medical Sciences (CIOMS), the exclusion of minors from clinical trials must be justified by robust scientific reasoning, ensuring that potential benefits are not overshadowed by concerns of vulnerability.
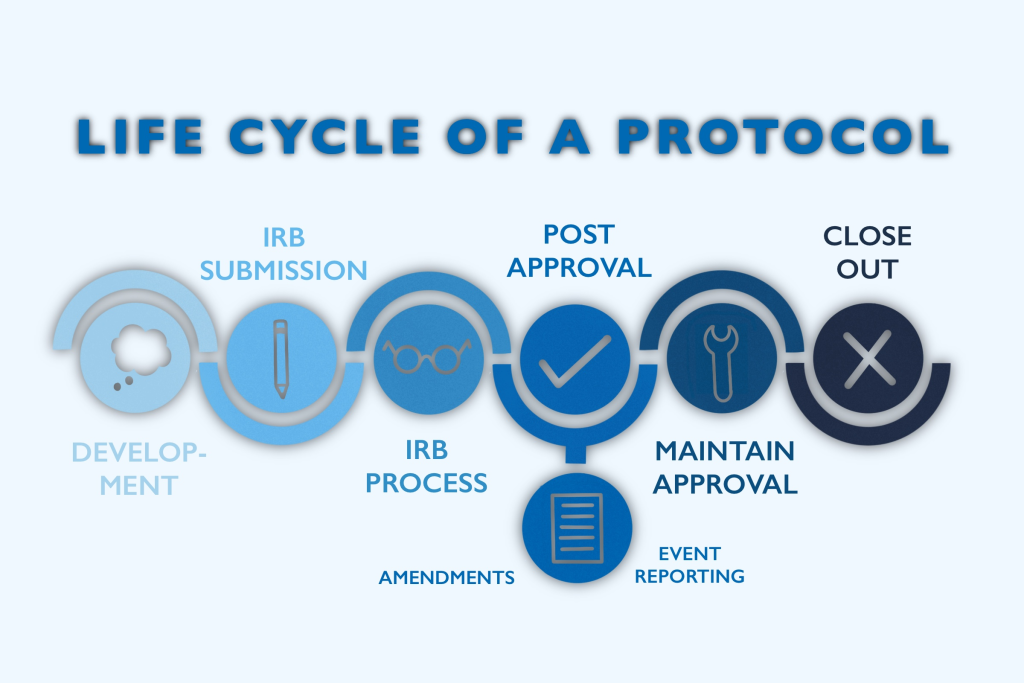
Balancing Risks and Benefits: A Delicate Equation
In clinical research, the assessment of risks and benefits assumes paramount importance, shaping the ethical contours of each trial. For minors, the stakes are elevated, as their capacity to comprehend complex medical information and provide informed consent is inherently limited. Here, the principle of minimal risk serves as a guiding beacon, dictating that the potential harms of research interventions must not exceed those encountered in everyday life. Moreover, the notion of direct and indirect benefits emerges as a crucial determinant, anchoring the ethical evaluation of clinical trials and underscoring the imperative of therapeutic efficacy and scientific advancement.
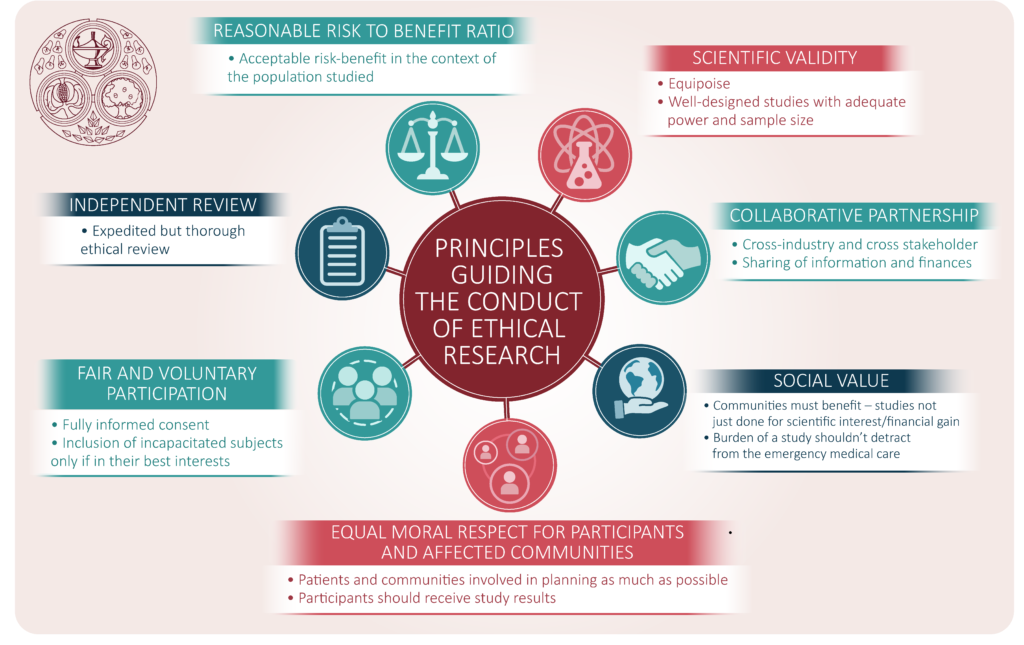
Upholding Research Integrity: The Role of Ethics Committees
Amidst the complexities of clinical research, the pivotal role of research ethics committees emerges as a safeguard against ethical transgressions. Tasked with meticulously scrutinizing research protocols, these committees serve as guardians of ethical acceptability, ensuring that the rights and well-being of minors remain paramount. Ethical review, coupled with robust scientific evaluation, forms the bedrock of research integrity, underscoring the ethical imperative to uphold the highest standards of professionalism and adherence to ethical principles.
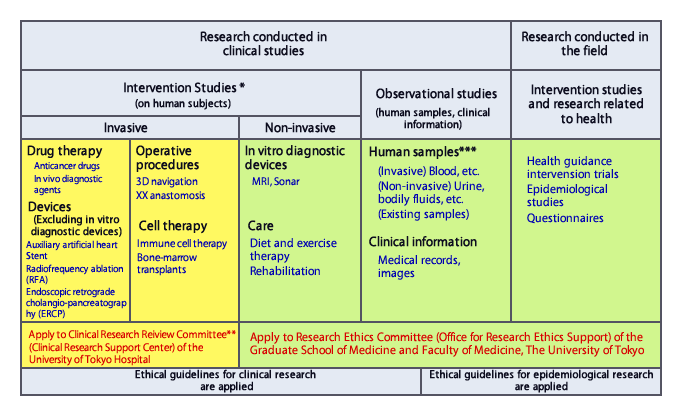
Cultural Diversity and Informed Consent: Navigating Multicultural Realities
Albeit the existence of such review committees, the intersection of clinical research with cultural diversity still poses nuanced challenges, necessitating a sensitive approach grounded in respect for diverse worldviews. Within multicultural societies, differing conceptions of health, illness, and autonomy complicate the process of obtaining informed consent, particularly among ethnic minorities. As emphasized by UNESCO’s International Bioethics Committee, the recognition of diverse cultural perspectives is essential, guiding researchers toward a nuanced understanding of individual autonomy within distinct cultural contexts.
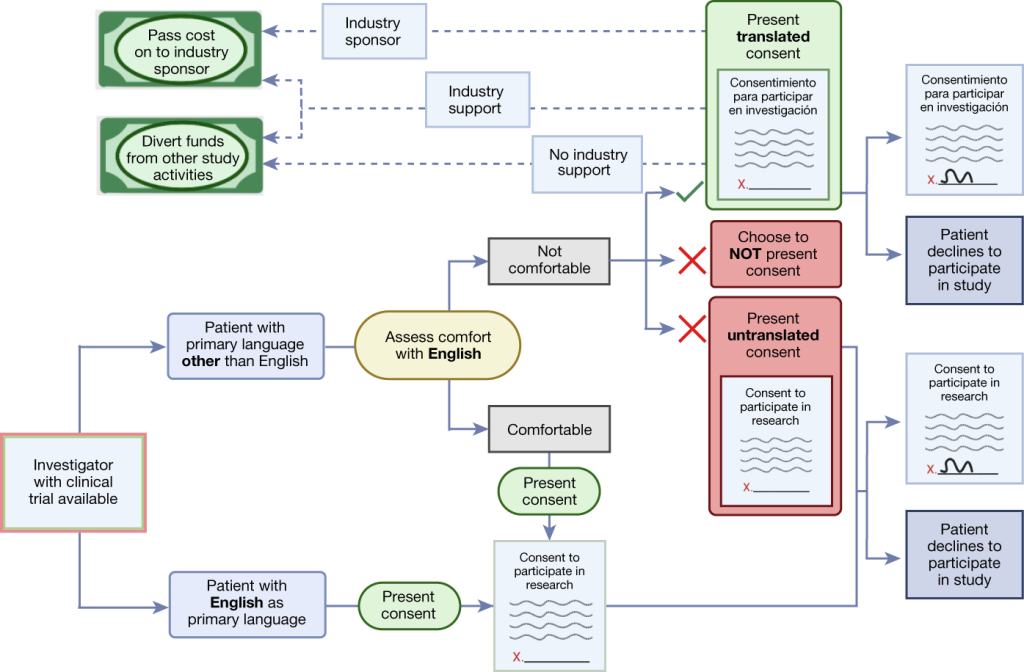
Empowering Autonomy: Evolving Role of Minors and Parents
At the heart of the ethical discourse on minors’ participation in clinical trials lies the intricate interplay between autonomy, maturity, and parental involvement. While minors occupy a legally defined status, their evolving capacity to understand and consent to research interventions underscores the dynamic nature of autonomy. Likewise, parental consent emerges as a cornerstone, balancing the rights of minors with the duty of parents to safeguard their well-being. However, as minors mature into adolescents, the transition towards independent decision-making heralds a shift in the ethical landscape, necessitating nuanced considerations of individual autonomy and parental involvement.
Clinical Trials: Always Anchored in Ethics
In the ethical discourse surrounding minors’ participation in clinical research trials, the imperatives of vulnerability, risk assessment, and autonomy converge, guiding researchers and ethicists toward a delicate balance between scientific progress and ethical responsibility. Rooted in principles of respect, beneficence, and justice, the ethical framework surrounding minors’ involvement in clinical trials serves as a testament to the enduring commitment to safeguarding the rights and well-being of the most vulnerable members of society. As medical science continues to advance, the ethical imperatives laid out herein will remain as guiding beacons, ensuring that the pursuit of knowledge remains firmly anchored in the principles of ethical integrity and human dignity.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts
Clinical Trial Supply Chain
Equitable Trial Strategy: A Comprehensive Blueprint for Diversifying Oncotherapeutics
In the pursuit of health equity, diversifying cancer clinical trials is not only a scientific imperative but a moral imperative.
Clinical Trial Supply Chain
Bridging the Gap: Enhancing Diversity in Cancer Clinical Trials
As cancer care marches forward, fostering diversity in clinical trials stands as a non-negotiable imperative.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.












