Major Depressive Disorder (MDD) stands as a formidable global health challenge, affecting a staggering 280 million individuals worldwide. The World Health Organization’s projections for MDD becoming the leading cause of disease burden by 2030 underscore the urgency of innovative therapeutic interventions. In a groundbreaking collaboration, Evecxia Therapeutics and Quotient Sciences have navigated the intricacies of drug development to unveil EVX-101, a promising drug candidate aimed at addressing the complex landscape of MDD.
Understanding Major Depressive Disorder
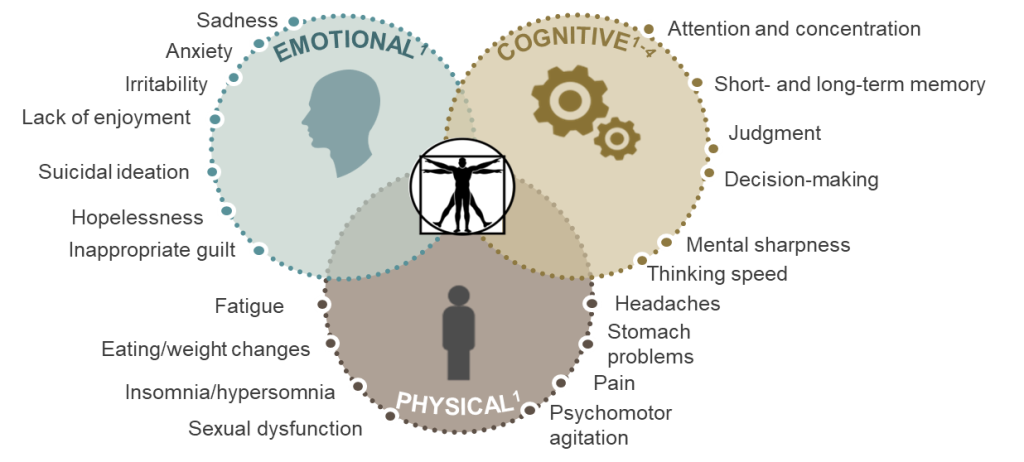
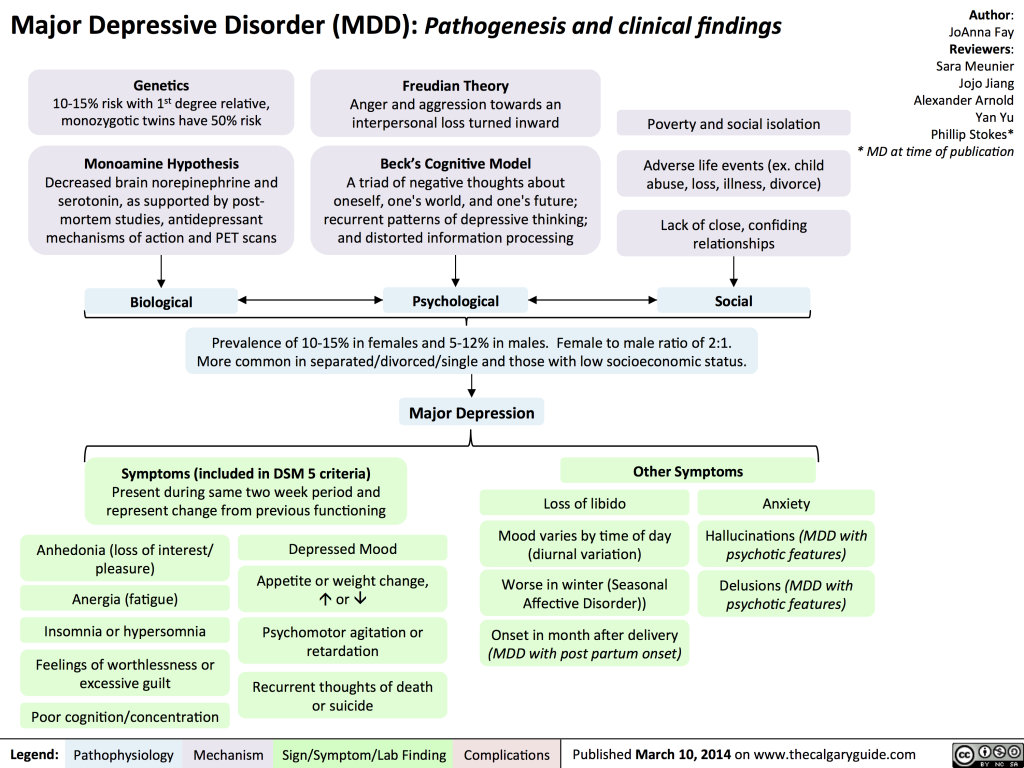
MDD, characterized by persistent low mood, anhedonia, and a range of associated symptoms – sensations of guilt or inadequacy, diminished energy levels, difficulties in concentration, alterations in appetite, psychomotor slowing or restlessness, disruptions in sleep patterns, or contemplation of suicidal ideation – poses a significant diagnostic and treatment challenge. According to the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5), a definitive MDD diagnosis requires the presence of five specific symptoms, including a depressed mood or anhedonia. Various forms of depressive disorders, such as persistent depressive disorder and premenstrual dysphoric disorder, further add to the complexity of MDD.

Exploring the Multifactorial Etiology
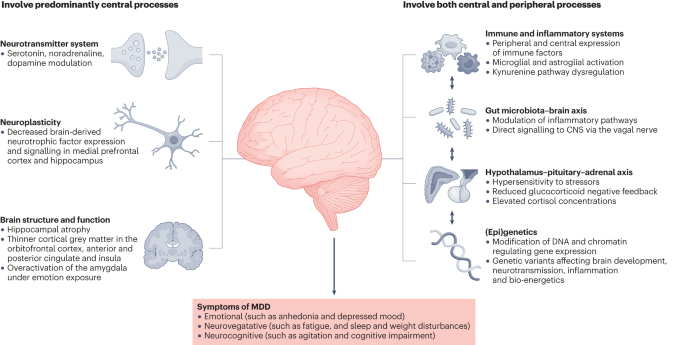
Delving into the etiology of MDD reveals a multifactorial interplay of biological, genetic, environmental, and psychosocial factors. While serotonin, norepinephrine, and dopamine were once the primary focus in understanding MDD, recent theories point to intricate neuroregulatory systems and neural circuits as key contributors. The involvement of inhibitory neurotransmitter GABA, excitatory neurotransmitters glutamate and glycine, and the impact of thyroid and growth hormonal abnormalities add layers to our understanding.
Epidemiology: A Pervasive Challenge
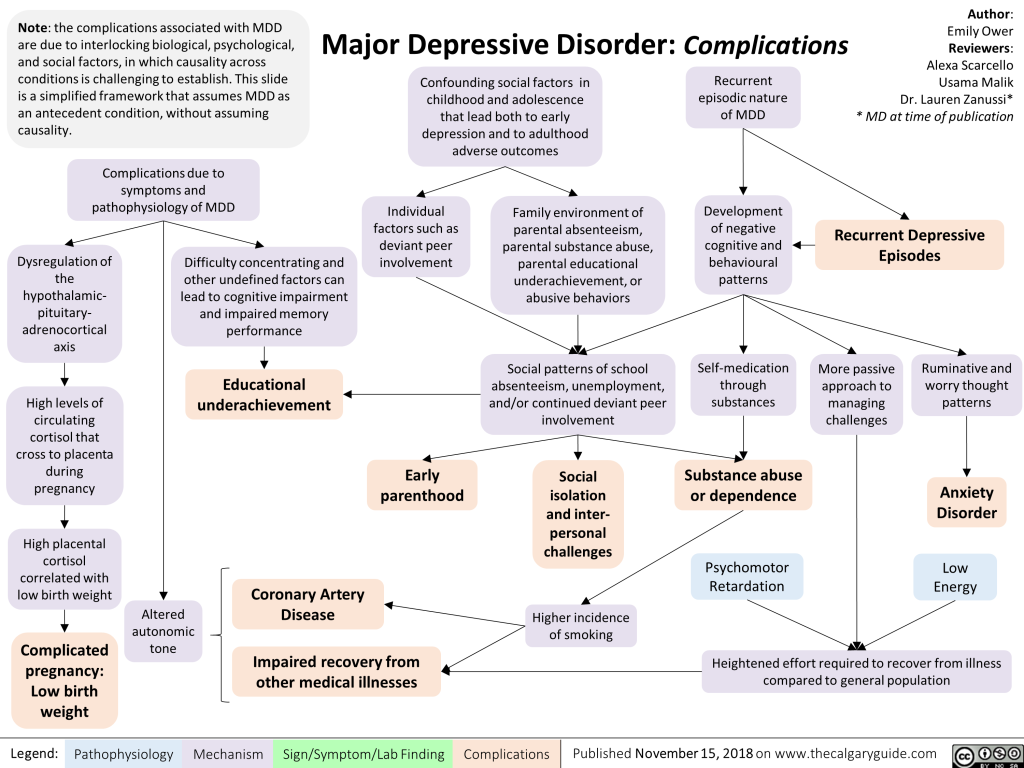
Major depressive disorder, with a lifetime prevalence ranging from 5 to 17 percent, imposes a considerable burden on global mental health. Gender differences, comorbidities, and the influence of interpersonal relationships contribute to the intricate epidemiological landscape. Evecxia’s dedicated efforts stem from the recognition that a significant portion of MDD patients do not respond adequately to first-line antidepressants, necessitating novel treatment approaches.

EVX-101: Pioneering Therapeutic Amplification
Evecxia’s pioneering approach involves amplifying serotonin synthesis, focusing on the novel drug candidate EVX-101. This gastro-retentive, sustained-release tablet formulation combines 5-hydroxytryptophan (5-HTP), the natural precursor to serotonin, with low-dose carbidopa, an absorption enhancer. Quotient Sciences’ Translational Pharmaceutics® platform played a pivotal role in optimizing the drug delivery mechanism, enabling the successful completion of Phase 1 clinical testing.

Quotient Sciences’ Acceleration: Bridging Innovation and Clinical Efficacy
The collaboration with Quotient Sciences, a drug development and manufacturing accelerator, significantly accelerated EVX-101’s development timeline. The Translational Pharmaceutics® platform seamlessly integrated formulation development, real-time manufacturing, and adaptive clinical study design, reducing costs and timelines. The Phase 1 trials, conducted with meticulous precision, showcased the safety, tolerability, and pharmacokinetic and pharmacodynamic data of the optimized EVX-101 formulation.
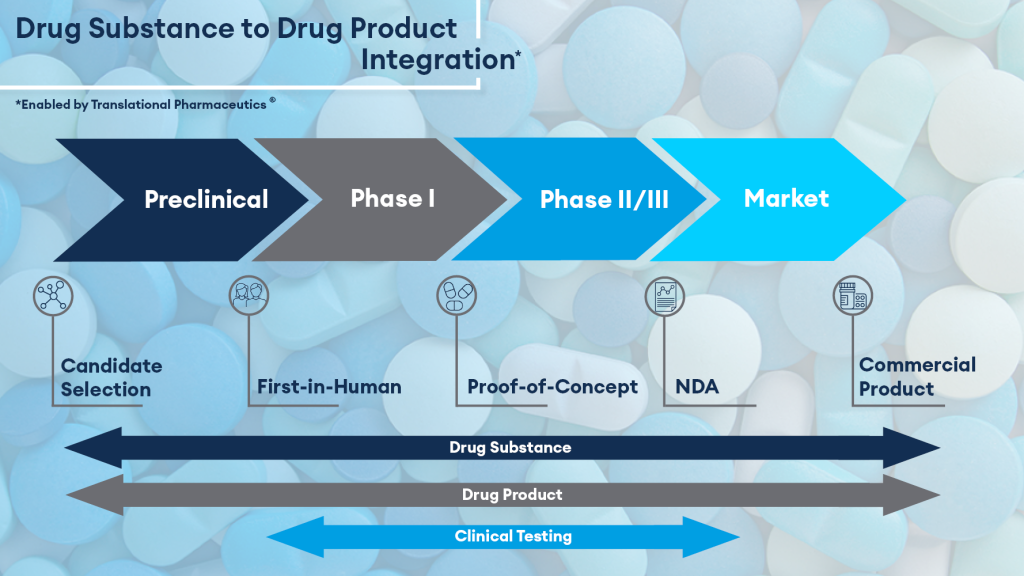
Moving Forward: Phase 2 and Beyond
With Phase 1 success in hand, Evecxia now embarks on a Phase 2 adjunctive treatment study targeting MDD patients who respond inadequately to first-line SSRIs/SNRIs. The collaborative efforts between Evecxia and Quotient Sciences underscore the imperative to expedite mental health treatment options. Jacob Jacobsen, Ph.D., CEO of Evecxia, expressed satisfaction with the condensed timeline, emphasizing the collaborative synergy between the two organizations. Mark Egerton, Ph.D., CEO of Quotient Sciences, echoed the sentiment, emphasizing the collective goal of advancing EVX-101 to offer new hope to those grappling with the life-limiting effects of MDD.
The journey of EVX-101 from collaborative conception to Phase 1 success not only signifies a major stride in MDD therapeutics but also exemplifies the potential of strategic partnerships to revolutionize mental health treatments. The intersection of innovative drug development and clinical precision paves the way for a brighter future in the battle against Major Depressive Disorder.
About Evecxia Therapeutics
Evecxia Therapeutics emerges as the pioneering entity dedicated to unlocking the therapeutic potential inherent in amplifying serotonin synthesis for the treatment of brain disorders. Setting itself apart from approaches targeting serotonin transporters (e.g., SSRIs) and receptors (e.g., psilocybin), Evecxia employs a unique strategy. The company utilizes 5-HTP, the natural precursor to serotonin, delivered through proprietary drug delivery technologies, ensuring sustained amplification of serotonin synthesis.
Currently boasting two Phase 2 clinical-stage drug candidates, Evecxia’s EVX-101 is undergoing development as an adjunctive treatment for depression when the efficacy of first-line SSRI/SNRI antidepressants alone proves insufficient. Simultaneously, EVX-301 is positioned as a rescue therapy specifically designed for patients experiencing acute suicidal ideation crises.
In terms of intellectual property, Evecxia holds a comprehensive portfolio comprising issued and pending patents. These patents cover aspects of the 5-HTP sustained-release/serotonin amplification method, various doses, formulations, and related technologies. This strategic approach underscores Evecxia’s commitment to advancing innovative solutions in the realm of brain disorder therapeutics.
Learn more about Evecxia from their website.
About Quotient Sciences
Quotient Sciences operates as a drug development and manufacturing accelerator, delivering integrated programs and tailored services throughout the entire development pathway. This organization navigates across diverse drug development capabilities, effectively streamlining processes to save valuable time and resources in bringing drugs to patients. The driving force behind all endeavors is a steadfast belief that ideas must evolve into solutions, and molecules must transform into effective cures swiftly, aligning with the urgent global need for solutions.
With a workforce exceeding 1,300 individuals, Quotient Sciences conducts its operations from cutting-edge manufacturing and clinical facilities in both the US and the UK. The strength of the organization lies in its dedicated team, fostering a distinctive workplace characterized by a passion for innovation and a commitment to revolutionizing drug development for the benefit of patients in need.
The recruitment strategy at Quotient Sciences centers on individuals who are dedicated to making a positive impact, demonstrating excellence in customer service, and displaying a readiness to go above and beyond. The organizational culture places a strong emphasis on teamwork, with a commitment to helping, supporting, and mentoring deeply embedded in its DNA. Prospective team members can anticipate joining an empowering and innovative environment that encourages continuous improvement and provides opportunities for learning and development on a daily basis.
Access more about Quotient Sciences via their official page.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Clinical Operations
Supervised Learning: Harnessing Data to Revolutionize Patient Care
As healthcare enters a new era, the integration of ML promises to revolutionize oncology and medicine.

Clinical Operations
The Interplay of Public and Private Sectors in Pharmaceutical Innovation
A delicate balance between public and private investments shapes the pharma R&D landscape.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.
Appreciating the Therapeutic Versatility of the Adenine Scaffold: From Biological Signaling to Disease Treatment
Researchers are utilizing adenine analogs to create potent inhibitors and agonists, targeting vital cellular pathways from cancer to infectious diseases.












