In the relentless pursuit of effective therapies to combat a myriad of diseases, the journey of drug discovery has evolved from serendipitous observations to a highly orchestrated and science-driven endeavor. At the heart of this quest lies the identification of precise molecular targets and the strategic assessment of their ‘druggability.’ The path from initial hypotheses to potential medicines is paved with questions and challenges that demand rigorous scrutiny and innovative solutions.
The Critical Role of Targets
Science Always Starts with an Intelligent Guess
Historically, the journey of drug discovery was often serendipitous, with researchers stumbling upon the effects of natural substances through empirical observations. However, a transformative shift has taken place in contemporary drug discovery methodologies. Instead of relying solely on chance discoveries, scientists now embark on a systematic and hypothesis-driven approach. This paradigm shift begins with a fundamental hypothesis: that a specific protein or biological pathway plays a pivotal role in the development or progression of a particular disease. This hypothesis-driven strategy not only streamlines the drug discovery process but also enhances its efficiency and success rate.
Crucial questions emerge from this hypothesis-driven approach. First, researchers ask whether it is possible to find or design a drug that can effectively modulate the chosen target, thereby altering its activity in a desired manner. Second, they investigate whether modifying the target’s activity has a meaningful impact on the course of the disease, improving patient outcomes. Lastly, the economic viability of the drug discovery endeavor becomes a pivotal consideration, as the costs associated with pharmaceutical research and development are substantial. The answers to these critical questions act as guiding principles, shaping the direction and intensity of drug discovery efforts. This strategic shift has not only accelerated the development of innovative therapies but has also improved the likelihood of translating scientific insights into tangible treatments for various diseases, ultimately benefiting patients worldwide.
Receptor and Enzyme Druggability
At the core of the drug discovery process lies the critical concept of ‘druggability.’ This concept hinges on the feasibility of effectively modulating a target with small organic molecules. To be considered druggable, a target must possess a binding site that exhibits not only considerable affinity but also a high degree of selectivity for the drug. Targets like enzymes and receptors for small ligands are often seen as promising candidates due to their well-defined binding sites and established interaction mechanisms. However, the landscape becomes more challenging when the target involves complex interactions with large peptides or proteins. In such cases, finding a precise drug-binding site becomes a daunting task. Additionally, accessibility is another pivotal factor in target selection, as extracellular targets are generally more amenable to drug intervention compared to intracellular ones, which are often shielded by cellular membranes.
Furthermore, the validation of a drug target cannot be overstated. Ensuring that a target plays a fundamental role in the pathogenesis of a specific disease is a critical step in the drug discovery process. Validation frequently relies on advanced molecular biology techniques and may entail the creation of animal models designed to mimic human biology closely. These models provide valuable insights into the target’s true potential and its relevance in the context of the disease being studied. Without robust target validation, the likelihood of failure in drug development significantly increases, emphasizing the importance of comprehensive and rigorous assessments before advancing potential drug candidates further in the pipeline. In essence, the interplay between druggability, accessibility, and validation forms the cornerstone of effective and successful drug discovery efforts.
What Can We Learn from the Hormone Leptin?
The concept of target validation serves as a crucial determinant in the drug discovery process, as it helps identify promising therapeutic candidates while avoiding potential pitfalls. A notable illustration of this principle lies in the discovery of the hormone leptin, which plays a pivotal role in the regulation of feeding and appetite. In the pursuit of obesity treatment, leptin initially emerged as a promising avenue due to observations in mice with mutations resulting in a loss of either leptin or its receptor, leading to extreme obesity. This indicated that leptin might be a potent tool in controlling obesity. However, the path to therapeutic success encountered a twist as further investigation revealed that obese individuals often have high levels of circulating leptin, but paradoxically, they appear insensitive to its appetite-suppressing effects. This example underscores the complexity of biological systems and the challenges in translating seemingly straightforward observations into effective drug interventions. It highlights the necessity of comprehensive target validation to ensure that a potential drug target is not only relevant but also amenable to modulation for therapeutic benefit.
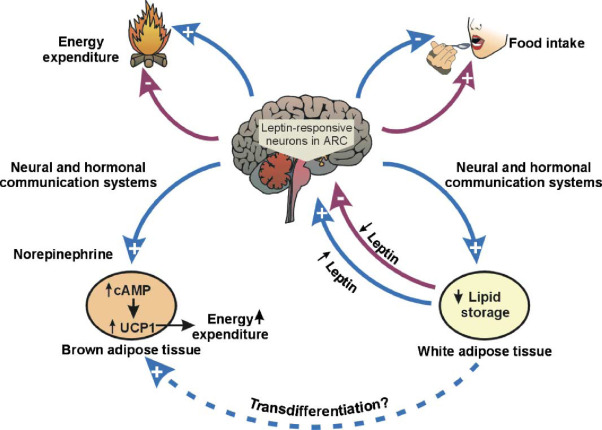
Biological systems frequently exhibit redundancy and compensatory mechanisms, particularly for functions deemed crucial for survival, such as appetite regulation. This complexity underscores the importance of in-depth target validation to navigate potential roadblocks in drug development. In cases like leptin, where the initial promise was met with unexpected challenges, a deeper understanding of the target’s biology became paramount. While the journey from target identification to therapeutic success may encounter hurdles, it is through rigorous validation and a keen awareness of biological intricacies that researchers can chart a more reliable path toward the development of effective drugs for complex conditions like obesity. This lesson on leptin serves as a valuable reminder of the intricate nature of drug discovery and the significance of thorough target validation in guiding successful therapeutic interventions.
Evaluating Economic Viability
How Financially Feasible is the Drug Invention Endeavor?
The economic landscape exerts a profound influence on the trajectory of pharmaceutical research and development. For investor-owned pharmaceutical companies, the primary consideration is the balance between the substantial costs associated with drug development and the anticipated returns on investment. This financial equation often dictates the areas of focus for these companies, leading them to prioritize diseases that affect larger populations and have the potential for greater financial returns. Rare diseases or those primarily afflicting economically disadvantaged regions may not align with the profit-driven model of these companies, making them less economically viable targets for drug development. In such cases, the pharmaceutical industry relies on alternative sources of support to address these unmet medical needs.
To bridge this gap, philanthropic organizations and governmental funding agencies play a pivotal role in driving research and development efforts targeting neglected diseases. Their support enables scientists and researchers to explore potential treatments for diseases that might otherwise be overlooked by the private sector. This collaborative approach, where public and private entities work together to tackle health challenges, has led to significant advancements in addressing neglected diseases, providing hope for underserved populations worldwide. Fundamentally, the economic considerations in pharmaceutical research underscore the importance of a multifaceted approach, where both market-driven incentives and external support mechanisms work in concert to advance drug discovery and ultimately improve global public health.
The Road to Potential Medicines
Additional Preclinical Research
Upon identifying a potential drug molecule that interacts with a validated target, the drug discovery process enters a critical phase of extensive preclinical research. This stage is characterized by a comprehensive evaluation of the molecule’s properties, addressing various facets that are essential for its development as a therapeutic agent. The properties under scrutiny include the molecule’s affinity and selectivity for the target, crucial factors that determine its effectiveness. Additionally, pharmacokinetics, which encompasses the molecule’s absorption, distribution, metabolism, and excretion (ADME) within the body, is meticulously assessed to understand how the drug behaves in vivo.
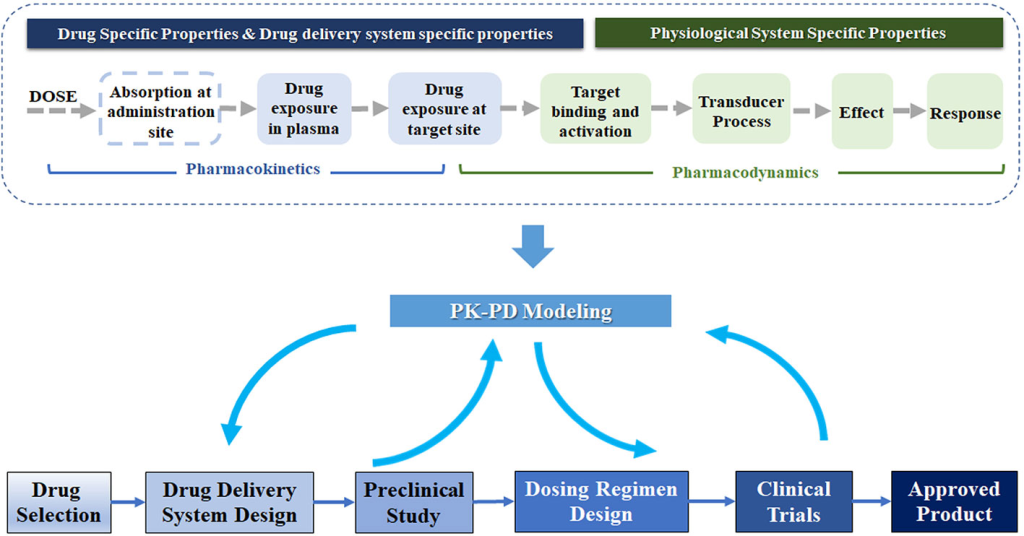
Furthermore, researchers evaluate the feasibility of large-scale synthesis or purification of the molecule, which is essential for producing sufficient quantities for clinical testing. Pharmaceutical properties, such as stability and solubility, are also taken into consideration to ensure that the drug can be formulated and administered effectively. Safety is paramount in drug development, and extensive testing is conducted to assess general toxicity, carcinogenicity, genotoxicity, and reproductive toxicity. A combination of animal models, including both rodents and nonrodents and in vitro assays is employed to evaluate these aspects. Importantly, researchers seek to discern whether any unwanted effects observed during these tests stem from interactions with the intended target (on-target effects) or arise from interactions with other unintended targets (off-target effects). This exhaustive preclinical research phase is critical in identifying potential safety concerns and optimizing the drug molecule before it progresses to clinical trials in human subjects.
The Regulatory Hurdle
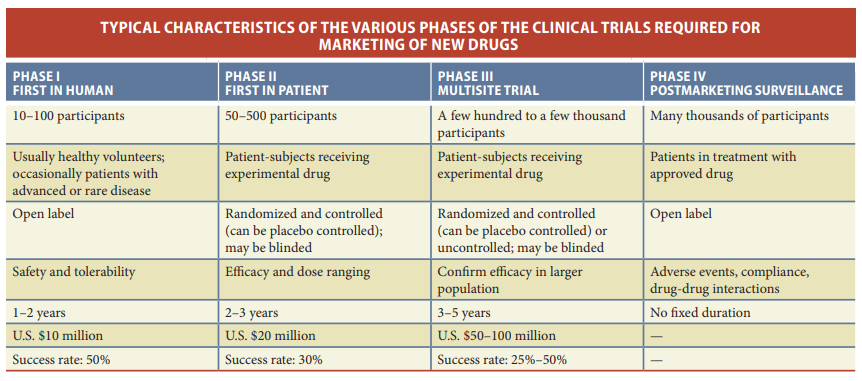
Before a drug candidate can advance to the crucial stage of clinical trials involving human subjects, it must navigate a complex and rigorous regulatory evaluation process. Central to this process is the submission of an Investigational New Drug (IND) application to regulatory agencies such as the U.S. Food and Drug Administration (FDA). The IND application serves as a comprehensive dossier, encompassing various essential elements that provide a detailed picture of the drug candidate and its potential for therapeutic use. This includes the scientific rationale behind the drug, preliminary evidence of its efficacy, in-depth pharmacological insights, extensive toxicological data, comprehensive chemistry and manufacturing information, and other critical details pertaining to the drug’s development and production.

The submission of the IND application marks a pivotal juncture in the drug development journey, as it represents the point at which the drug candidate moves from preclinical research into human research. Regulatory agencies like the FDA play a central role in reviewing and evaluating the application. They have a 30-day window to assess the IND, during which they may disapprove the application, request additional data, or grant permission for the drug’s initial clinical testing to commence. This regulatory oversight ensures that the drug’s safety and efficacy are thoroughly scrutinized before it is administered to human subjects, emphasizing the paramount importance of adherence to rigorous scientific and ethical standards in the pursuit of novel therapeutics.
As we traverse the intricate landscape of drug discovery, these pivotal steps underscore the meticulous and scientific approach that drives our pursuit of novel medicines. The fusion of cutting-edge science, innovative hypotheses, and economic considerations is shaping a future where the most challenging diseases may one day yield to the power of targeted therapies.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Drug Discovery Biology
Unveiling the Elusive: The Prevalence Problem in Drug Discovery
When it comes to developing new drugs, researchers encounter a formidable challenge: the prevalence problem.

Drug Discovery Biology
Navigating Drug Development: Innovations in Preclinical Testing
As we navigate the complexities of drug development, a harmonious blend of traditional wisdom and innovative technologies will be paramount.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.
Appreciating the Therapeutic Versatility of the Adenine Scaffold: From Biological Signaling to Disease Treatment
Researchers are utilizing adenine analogs to create potent inhibitors and agonists, targeting vital cellular pathways from cancer to infectious diseases.
Bioavailability and Bioequivalence: The Makings of Similar and “Close Enough” Drug Formulations
Scientists are striving to understand bioavailability complexities to ensure the equivalence of drug formulations from different manufacturers, crucial for clinical effectiveness.