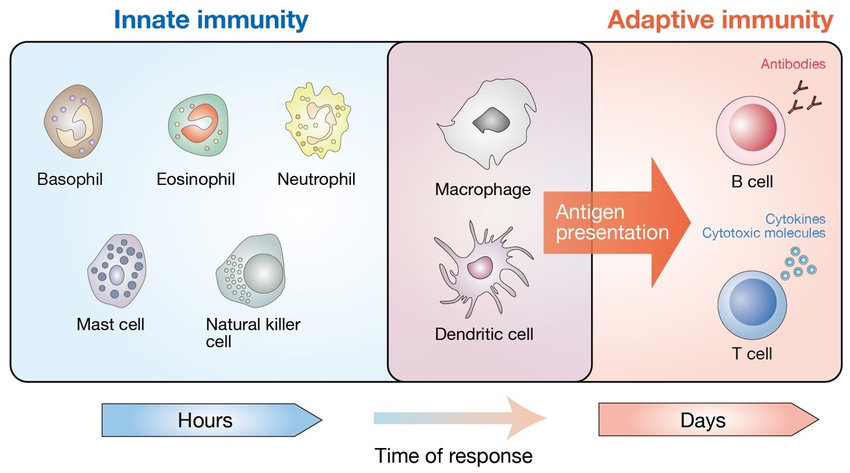
In a recent review article published in Cell Host & Microbe, scientists from the National Institute of Allergy and Infectious Diseases (NIAID), a division of the National Institutes of Health (NIH), examine the difficulties and present solutions for better vaccinations. Together with Jeffery K. Taubenberger, M.D., Ph.D., and David M. Morens, M.D., the article’s authors include former NIAID director Anthony S. Fauci, M.D.
Doubling Down on Differences
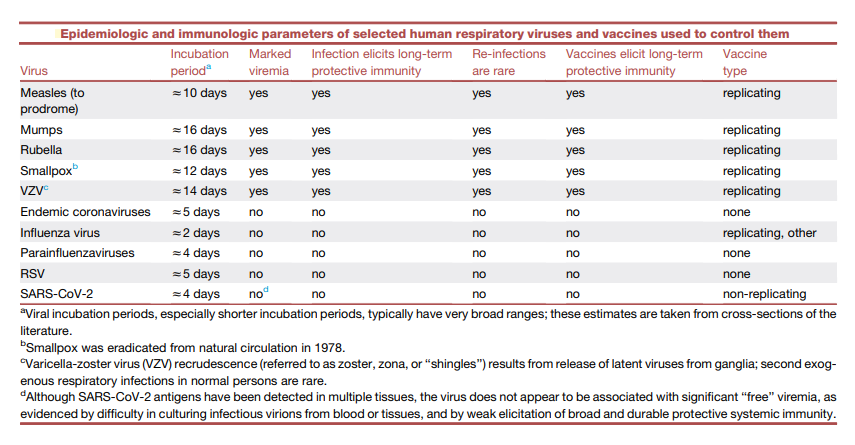
There was anticipation that vaccines for all other respiratory viruses would soon be created more than 50 years ago with the successful creation of vaccines against some of the most significant respiratory viruses, such as measles, mumps, and rubella (MMR). However, natural infections with these three respiratory viruses that can be managed by vaccination, as well as smallpox and varicella zoster virus (VZV), are not typical of infections brought on by the majority of respiratory viruses. To successfully manage them with vaccines, they differ in at least three very significant ways (see the table below):
(1) Following mucosal replication, all of these systemic respiratory viruses induce considerable viremia, which disperses a vast number of infectious virions throughout the body and exposes them to a variety of immunological compartments and immune competent cell types,
(2) They exhibit unusually lengthy incubation times that represent the initial mucosal replication and subsequent systemic dissemination of infectious virions, allowing enough time for the induction of the full force of adaptive immunity, and
(3) They produce a lifelong or persistent protective immunity.
However, non-systemic respiratory viruses like influenza, SARS-CoV-2, and RSV tend to have considerably higher transmission rates than systemic respiratory viruses. Less time is spent incubating (see the table above) and viral replication proceeds quickly. They mostly reproduce in local mucosal tissue, without viremia, and without much interaction with the systemic immune system or the full force of adaptive immune responses, which take at least 5-7 days to mature, frequently after the peak of viral replication and subsequent transmission to everybody else. RT-PCR levels of viral RNA have been linked to severe disease, similar to studies of influenza RNAemia. SARS-CoV-2 “RNAemia” (circulation of viral RNA in the bloodstream, as is seen with most mucosal respiratory virus infections, as distinct from viremia, in which infectious viruses can be cultured from the blood) has been reported. As a result, non-systemically replicating respiratory viruses, including SARS-CoV-2, seem to re-infect people over the course of their lifetimes on a regular basis without ever producing fully effective and long-lasting immunity.
Although RNA viruses have a common inherent RNA-dependent RNA polymerase error rate, various viruses (and different open reading frames within their genomes) have variable levels of mutagenic tolerance. This is an essential additional consideration. Frequently overlapping open reading frames or functional restrictions on the acquisition of nonsynonymous mutations, as is the case, for instance, with the measles virus, can both be related to mutational constraints. The hemagglutinin and neuraminidase proteins of the external influenza A virus, however, are relatively plastic, and positively selected nonsynonymous mutations cause immunologically significant antigenic drift by acquiring nonsynonymous mutations in antigenic epitopes and by changing the N-linked glycosylation patterns. The development of broadly protective, “universal” influenza vaccines is made more difficult by rapid antigenic drift, which also affects the management of yearly influenza epidemics. With the advent of several variations with changing antigenicity, the SARS-CoV-2 spike protein has demonstrated a similar plasticity, making it more difficult to manage with current immunization techniques.
Other mucosal-only respiratory viruses, like RSV, have shown much less antigenic plasticity, but they still cause repeated infections over the course of a lifetime without the development of long-term protective immunity. Although rapid evolution of antigenically variable mucosal viruses, like influenza A viruses and SARS-CoV-2, complicates next-generation vaccine design. The lack of induction of long-lasting protective immunity against other respiratory mucosal viruses, such as the more phenotypically stable RSV, cannot entirely be attributed to genetic and antigenic variability, even though these factors make vaccine design more difficult for viruses like influenza and SARS-CoV-2.
Given all of these aspects, it is not unexpected that vaccinations have never been able to successfully control any of the respiratory viruses that primarily affect mucous membranes. This finding raises a crucial question: how can we expect vaccinations, particularly systemically delivered non-replicating vaccines, to provide comprehensive and long-lasting protective immunity against reinfection? Natural mucosal respiratory virus infections do not. The creation of future vaccines faces a significant hurdle, and overcoming it is essential as we try to create “next-generation” vaccinations.
The search for new and improved respiratory virus vaccines is covered herein along with some of the major problems, which are listed in the table under.
Immune Tolerance Endows Immunity Against Immune System
Because the human immune system has evolved to tolerate them during the extremely brief periods of mucosal viral replication, natural infections with mucosal respiratory viruses may not be completely controlled by human immune responses.
The signs and symptoms of numerous different mucosal respiratory virus infections are very similar: a brief illness with a relatively straightforward course that includes rhinorrhea, sneezing, sore throat, varied cough, malaise, and frequently mild or absent temperature. These similarities clearly imply that these viruses share comparable pathogenic pathways involving human inflammatory and innate immune responses.
Numerous variables, including the evolution of the virus and the host, contribute to the mucosal respiratory viruses’ incapacity to elicit a long-lasting protective immunity. The human respiratory immune system has evolved to express complex tolerance regulation, which may inhibit effective viral immune elimination during the first 5-7 post-inoculation days, when innate immune responses predominate. Additionally, very short incubation periods allow substantial and unchallenged viral rep.
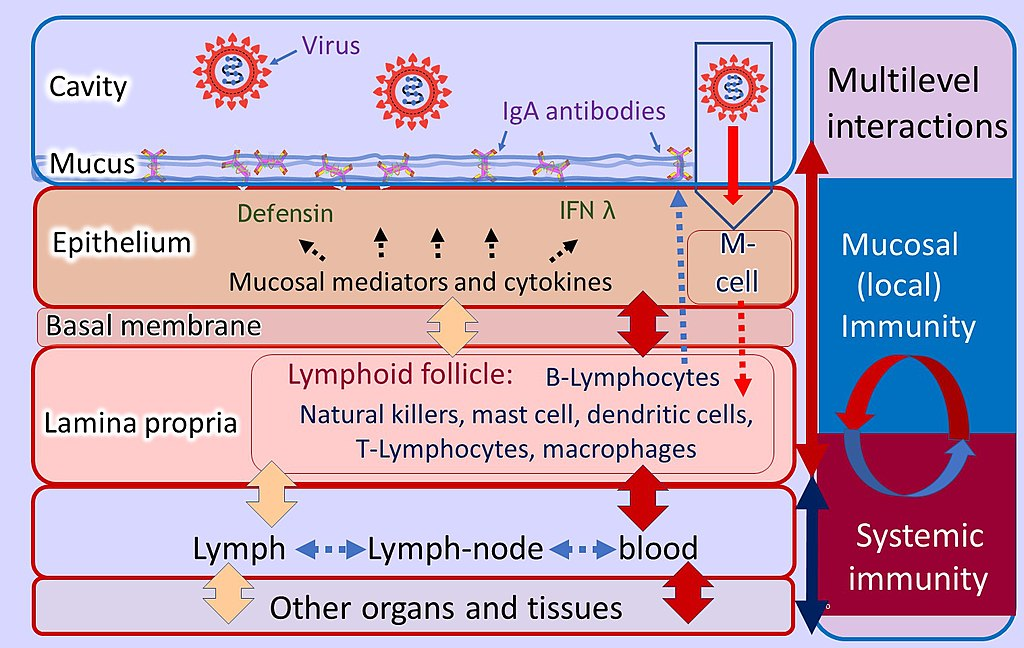
Furthermore, compared to immune system compartments in other organ systems, the respiratory immune system’s many compartments each perform in a very different manner. The semi-organized, mucosal-associated lymphoid tissues (MALTs) of the tear/conjunctival associated lymphoid tissue (TALT), nasopharyngeal associated lymphoid tissue (NALT), and bronchial associated lymphoid tissue (BALT), as well as in distinct pulmonary compartments, are where the respiratory immune system is specifically located. Each of these compartments separately detects viral infection and antigen presentation, communicates with the other compartments and the systemic immune system, starts local effector responses, and keeps a variable immunological state.
Accessing Advantages of Alternative Host Immune Mechanisms
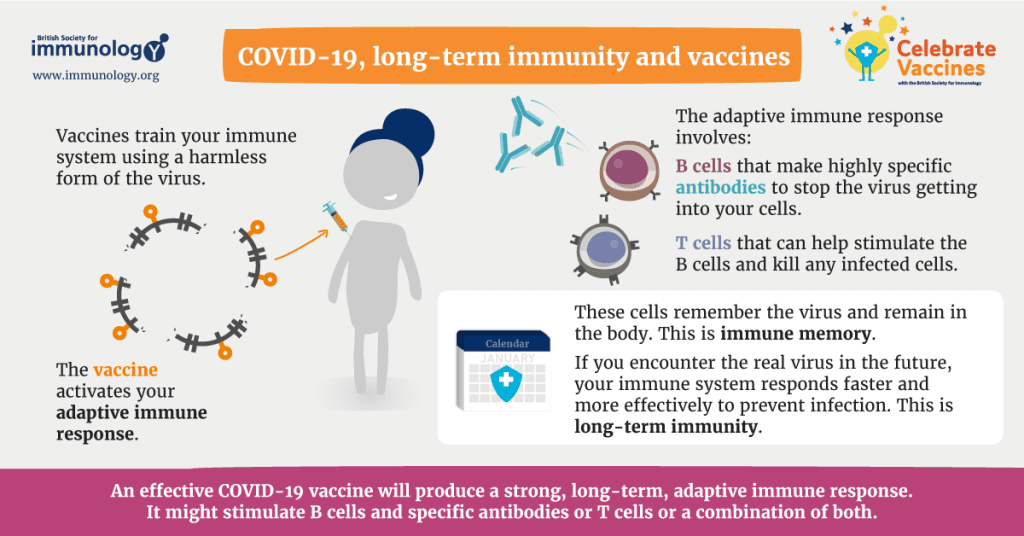
We must utilize other host immune mechanisms since mucosal and systemic immunity only partially protects against infection with mucosal respiratory viruses. For instance, the phenomena of “trained innate immunity,” which has just lately come to light, holds out hope that one day, vaccinations may be able to enhance innate immune responses, either specifically or more generally. How to manage these reactions to prevent damaging inflammatory effects, however, remains a crucial unresolved subject.
However, neither circulating antibodies, plasmablasts, nor systemic B, T, or T effector cells perform well at mucosal sites despite non-systemically replicating mucosal viruses eliciting systemic effectors, including systemic IgA-producing plasma cells and, in some cases, high levels of serum IgA and IgG. Because many of these effector cells lack trafficking signals to these sites and because transuded antibody has been diluted, this is partly the result.
Understanding the “crosstalk” between the upper respiratory, lower respiratory, and systemic immune systems, at the level of cell receptors, antigen sensing, antigen presentation, and numerous effector functions, will help us understand how vaccines might protect against lower respiratory infections. Further research is needed to understand how to maintain resident memory B and T lymphocytes specific to viruses in the lung, how to prolong their survival there, and how rapidly they may be deployed to infected mucosal locations.
Viral Strains, Viral Subtypes, Viral Drifts, and Inter-individual Variation
Next-generation vaccines will require the identification of robust immunologic correlates of protection against each mucosal respiratory virus, as well as consensus on their applicability to public health vaccination objectives. It is obvious that more immunological correlate studies in people are required and should be a top research focus. Following influenza infection in humans, research have long established serum and mucosal immunoglobulin correlates and T cell immunological correlates. In contrast, no immunologic correlates of protection were discovered in a human influenza challenge research conducted after vaccination with inactivated vaccines or live-attenuated influenza vaccine (LAIV), followed by LAIV challenge.
The immune system is intricate and has numerous effectors. Due to associations with other more important (but rarely measured) immune effectors, such as mucosal immunoglobulins or, for example, steric interactions of hemagglutinin stem antibodies resulting in neuraminidase inhibition, serum antibody titers to various viral epitopes may only weakly correlate with protection. Comparatively to hemagglutinin head or stem antibody titers, serum neuraminidase antibody titers were more highly linked with several indices of protection in recent human challenge experiments. Neuraminidase remains and undervalued vaccine target for next-generation influenza vaccine design. In conclusion, although statistically valid in large studies, correlations between serum antibody titers and susceptibility to influenza infection may be imprecise due to individual diversity, rapid virus evolution, and declining titers.
We must also agree on the desired levels of protection for each mucosal virus. For example, goals for protection might be (1) preventing infection completely, as vaccines for systemic respiratory viruses may do, (2) limiting viral replication or preventing transmission, as with influenza anti-neuraminidase immunity, (3) disease prevention, or (4) preventing only respiratory diseases that require hospitalization as with serious cases of influenza and SARS-CoV-2.
To create the finest vaccines, as well as the most effective immunization tactics and regulations, such consensus is required. For instance, vaccinations for influenza have traditionally been made to prevent upper respiratory infections rather than secondary pulmonary infections brought on by transmission from the upper respiratory tract. This has been an issue because the effectiveness of the current influenza vaccinations in both avoiding infection and inducing pulmonary immunity is subpar. Tens of thousands of people die from influenza each year in the United States, despite the fact that influenza and SARS-CoV-2 vaccines can lessen the severity of the disease when they fail to prevent infection. Given the flaws in current vaccines, it is urgent for public health to actively seek for better vaccines and immunization methods.
Asking The Right Questions for Vaccine Development
About 30 to 40 square feet of human mucosal surfaces are covered in active lymphoid tissues. The most prevalent sIgA (secretory form of immunoglobulin A), which accounts for an astounding 65%–70% of all human immunoglobulins, is the antibody at the majority of these locations. It is well acknowledged that the method of vaccine administration—such as intramuscular, intranasal, conjunctival, or aerosol—is a significant factor in determining the mucosal respiratory response.
Mucosal immunization appears to be the best strategy for respiratory viruses in general and when practical, but when thinking about next-generation vaccines we may also need streamlined formulations, elevated vaccine doses, increased frequency of vaccine administration, and combating immune tolerance challenges.
It is important for each virus to answer key questions such as: (1) Can non-replicating vaccinations, which may be far less successful at inducing IgA, be as effective as replicating vaccines, such as live vaccines containing attenuated viruses and live vaccine vectors expressing essential viral proteins? (2) Can vaccinations that contain a single or few antigens offer protection on par with those that contain more complicated antigens? (3) Can repeated vaccinations or larger antigen doses produce improved immunity? (4) How do soluble vs particulate antigens differ in their effects? (5) How should vaccine antigen load and systemic or mucosal adjuventation be correlated? (6) What are the best methods for administering vaccinations—mucosal/systemic “prime-boost”—and when should they be given? Newer techniques like “prime-pull” and “prime-deploy” (vaccination tactics that elicit systemic T cell responses followed by activating T cell recruitment via attractant or activating resident memory T cell recruitment to lung, respectively) and others?
All of these thought-provoking issues should be taken into account as we strive to improve vaccination tactics. Making vaccines that promote innate immunity is an intriguing direction to go in, as mentioned above. These vaccines might be ideal for preventing “hit and run” infections by mucosal respiratory viruses, which typically infect, spread locally, and spread to others before adaptive immune responses that can control them can be mounted. The choices for mucosal vaccination adjuventation are currently limited. If evidence suggests that mucosal vaccine adjuventation is required and practical, we must consider any drawbacks, including potential toxicity and adverse consequences of repeated high-dose mucosal immunization/adjuvantation, as well as the most effective way to conduct human studies.
Forward to a Failure-Proof Future
Vaccine development efforts have so far failed to produce long-lasting vaccinations against non-systemic mucosal respiratory viruses with substantial mortality rates. The development of next-generation respiratory vaccines has numerous, intricate challenges, as mentioned above. If we are to rationally create vaccines that prevent them, we must better comprehend why repeated sequential mucosal infections with the same circulating respiratory viruses, dispersed over decades of life, fail to elicit natural protective immunity, particularly with viruses that lack significant antigenic drift (e.g., RSV and parainfluenzaviruses). To create next-generation vaccines that induce immune defense against viruses that survive in human populations due to their capacity to dramatically evade the whole protective range of human innate and adaptive immunity, we must think outside the box.
Mucosal respiratory viruses have caused lethal epidemics and pandemics, and previous futile attempts to control these outbreaks and pandemics have been a scientific and public health disaster that has to be immediately addressed. Researchers and collaborative teams are currently reevaluating all of our prior theories and methods for preventing serious respiratory virus illnesses from the ground up in an exciting and energizing effort to forge brave new directions.
Subscribe
to get our
LATEST NEWS
Related Posts
Infectious Diseases & Vaccinology
Rezzayo™’s Latest EU Approval for Invasive Candidiasis Breaks Ground in Antifungal Therapy
Rezafungin marks the initial addition to the treatment arsenal for patients grappling with invasive candidiasis in more than 15 years.
Infectious Diseases & Vaccinology
Unmasking the Shadow: CDC Battles the Latest Fungal Meningitis Outbreak in Matamoros, Mexico
CDC tackles fatal fungal meningitis outbreak linked to surgeries in Matamoros, Mexico.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.