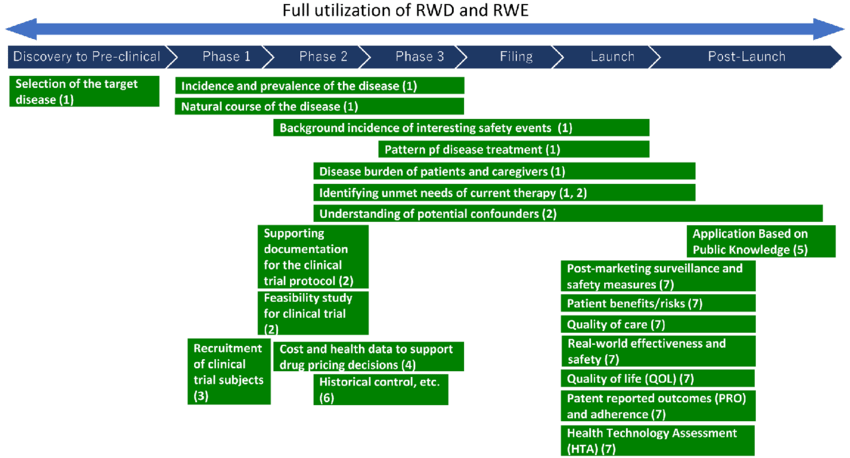
Clinical outcomes are better achieved when real-world evidence (RWE) is utilized in both the formulation of criteria for clinical trials and the optimization of patient recruitment. Biotechnology companies and pharmaceutical corporations alike agree that not only are clinical trials expedited, but the costs of holding every phase of the trial are significantly reduced when RWEs are opted than randomized control set-ups. This goes without saying that the utility of real-world data (RWD) allows companies to establish novel opportunities for patient engagement and development of superior drug products for public use.
According to AbbVie‘s Vice President and Global Head of Health Economics and Outcomes Research, Christopher Boone, PhD, FACHE, FHIMSS, the pharmaceutical and biotech industry is at the golden age of discovering novel use cases for how real-world data (RWD) are applied to produce real-world evidence (RWE) for drug development research.
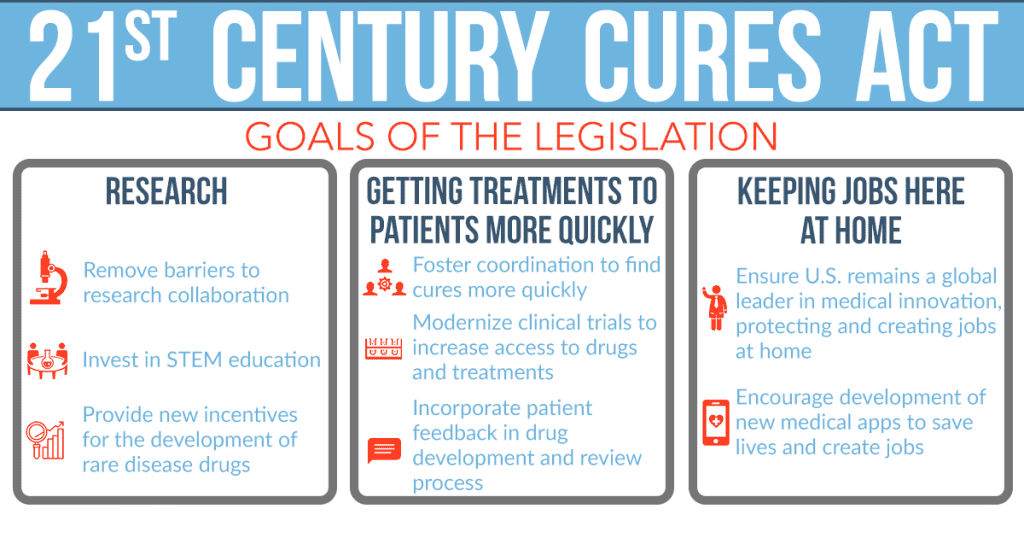
The 21st Century Cures Act
These past few years had seen incremental efforts from pharmaceutical companies by allowing RWE to guide the pre-clinical and clinical trials they hold. In addition, the United States Food and Drug Administration (US FDA) took this to heart and launched the 21st Century Cures Act. Introduced in the House as H.R. 34 by U.S. Representative for Oregon’s 1st Congressional District Atty. Suzanne Marie Bonamici on January 6, 2015, the “[An] Act to accelerate the discovery, development, and delivery of 21st century cures, and for other purposes” was signed into law by 44th POTUS Barack Hussein Obama II on December 13, 2016.
The regulation is intended to aid in accelerating the development of medical products and get new discoveries and advancements to people who require them more quickly and effectively.
The Cures Act expands on the continuing efforts of the US FDA to consider the viewpoints of patients when developing new medications, biological products, and devices. Our ability to update clinical trial designs, such as the utilization of real-world evidence and clinical outcome assessments, is improved by The Cures Act. This will hasten the development and evaluation of novel medical products, such as medical countermeasures.
Additionally, it establishes new expedited product development programs, such as (a) the Regenerative Medicine Advanced Therapy, or RMAT, which provides a new expedited option for some eligible biologics products, and (b) the Breakthrough Devices program, which is intended to expedite the review of some novel medical devices. It also grants the FDA new authority to assist in improving our capacity to hire and retain scientific, technical, and professional specialists.
Furthermore, the Cures Act instructs the FDA to establish one or more intercenter institutes to facilitate coordination of efforts in key disease areas between the drug, biologics, and device centers and enhances the regulation of combination products.
When Shorter Timelines Made More Sense
According to Dr. Boone, for the increased usage of RWE, particularly on the R&D side of the house, the 21st Century Cures Act was perhaps the single biggest impetus. The industry now has the freedom to come up with creative ideas for how we may use RWE in the future. One of the best examples – vaccination, specially COVID-19 vaccination.
There are safe and efficacious vaccines that offer excellent defense against COVID-19-related significant disease, hospitalization, and death. The COVID-19 vaccine has already been administered to billions of people. One of the most crucial things you can do to protect yourself from COVID-19, aid in the end of the pandemic, and prevent the emergence of new versions is to be vaccinated. The current COVID-19 vaccinations offer excellent defense against the Omicron and Delta forms of the virus that causes COVID-19, which can cause severe disease and even death. Complete vaccination will also lessen the possibility of new variations surfacing.
Dr. Boone cites the COVID vaccination as an indirect result of the Cures Act, attributing a large part of the rapid development of the vaccine to RWE. Thanks to the Cures Act’s impact on the clinical scene, scientists were able to reduce what had previously taken 10 to 15 years to only a few months.
Data – The Major Tool for Disruptive Innovation
The gold standard for drug discovery and development will always be the evidences – pharmacologic data, pharmacokinetic profiles, ADME, ADEs, ADRs – obtained from clinical trials. However, it is too narrow-minded to think of them as the only guiding principles in the future drug product endeavors of pharmaceutical and biotechnological industries.
Alternatively, RWE influences the choices made by well-known corporations, consumers, payers, doctors, and almost all other industry stakeholders. Dr. Boone weighs in on RWE as a “disruptive innovation”. He believes it has the potential to upend current practices in drug research and development as well as the way many medicines are commercialized.
He claims that without RWE, the advancement of digital technology, and the emergence of artificial intelligence and machine learning as powerful analytics instruments, the industry as a whole would not be able to move toward more collaborative, autonomous, and community-based clinical trials.
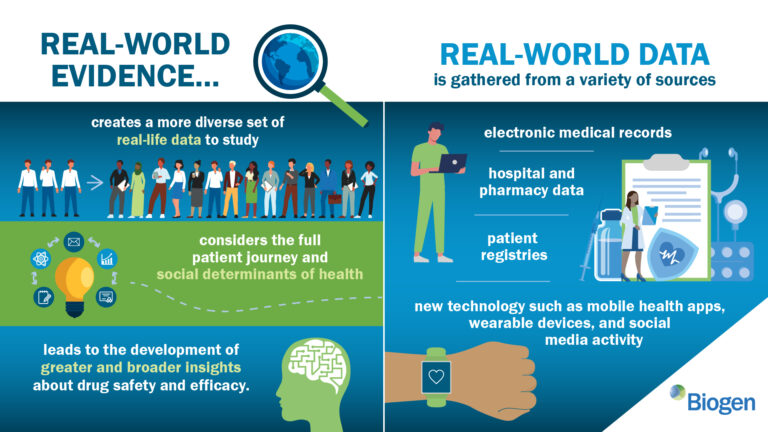
Additionally evolving are the accepted techniques for data curation. Due to the development of health data platforms and patient-generated data repositories, RWE makes it possible to conduct numerous interdisciplinary investigations using a single data set. The majority of the data that matters to us is collected outside of the provider environment. With today’s advanced digital technology, it is possible to comprehend the patient’s surroundings and experiences in real time.
Final Pieces of the Clinical Trial Puzzle
Employing a “learning-based culture,” in which businesses embrace a curious approach to drug development with a readiness to be flexible and learn new things, is one of the last hurdles, in Dr. Boone’s opinion, to the broad adoption of RWE. For the majority of pharmaceutical companies, this strategy is undoubtedly difficult.
RWE participation in clinical research also poses new problems for patients. People are becoming more wary of how and why their data is utilized as corporations gather more RWD from a variety of sources. As a result, the majority of life science companies are compelled to change how they handle data, especially how they perceive data security and privacy from the standpoint of the patient.
As the industry pushes for more patient-centric trials, it is critical that stakeholder mindsets, corporate cultures, and trial design adjust to accommodate the expanding usage of RWE. And professionals like Dr. Christopher Boone are dedicated to changing clinical research on all fronts, including how the goods produced by RWE are marketed and judged to be profitable for their separate parent firms.
Subscribe
to get our
LATEST NEWS
Related Posts

Clinical Operations
Supervised Learning: Harnessing Data to Revolutionize Patient Care
As healthcare enters a new era, the integration of ML promises to revolutionize oncology and medicine.

Clinical Operations
The Interplay of Public and Private Sectors in Pharmaceutical Innovation
A delicate balance between public and private investments shapes the pharma R&D landscape.
Read More Articles
Navigating the Molecular Pathway: Small Molecule Drug Discovery from Hit Identification to Clinical Development
The drug discovery process, particularly for small molecules, follows a well-defined path that aims to ensure efficacy, safety, and developability.
From Hit to Human: The Evolving Science of Drug Discovery
The transition from traditional small molecule drugs to biologics underscores the growing sophistication of the field.
From Traditional Drug Metabolism to Mechanistic Precision: The Evolution of Lead Discovery in Modern Pharmacology
The pursuit of mechanistic understanding has advanced the field far beyond simple pharmacokinetics.
Rethinking Preclinical Models in Drug Discovery
The design of new preclinical models should carefully consider both their biological relevance and practical feasibility.
From Mice to Man: Revolutionizing Drug Discovery with Human-Based Models
As we move toward a future where personalized medicine becomes the norm, the development of human-relevant preclinical models will be critical in bridging the gap between bench and bedside.
Navigating the Complex Evolution of Preclinical Models
The evolution from traditional to modern lead discovery represents a pivotal moment in drug development.
Modern Lead Optimization: A Journey through Science and Innovation
As traditional drug discovery merges with modern computational approaches, the future of pharmacology looks promising.
From Serendipity to Strategy: The Evolution of Drug Discovery
The fusion of traditional drug discovery methods with modern technologies marks a new era of drug development.