Carbohydrates serve as the fundamental metabolic building block for the synthesis of nearly all microbial products, although this category of antibiotic drugs is limited to those that are generated directly from recognized carbohydrate precursors. Amikacin, gentamicin, kanamycin, neomycin, netilmicin, paromomycin, spectinomycin, streptomycin, and tobramycin are among the therapeutically effective antibiotics generated from carbohydrate metabolism.
Chemical Overview
These antibiotics have comparable chemical and biologic properties. Water solubility, a strong basic character, and stability are typical chemical characteristics. With the exception of spectinomycin, the antibiotic compounds typically include 2 to 3 unusual sugars connected glycosidically to an amino-substituted cyclohexanyl aglycone. Aminoglycoside antibiotics is a term used to refer to these substances generally. Antibiotic combinations of closely related compounds are typically created through fermentation, making it impossible and useless to separate the individual components for therapeutic use. Due to the fact that they are semi-synthetic substances made from sisomicin and kanamycin A, respectively, amikacin and netilmicin differ from one another. Spectinomycin is an aminocyclitol derivative, not an aminoglycoside, although it shares many of the same characteristics with antibiotics that are aminoglycosides.
Notable Adverse Effects
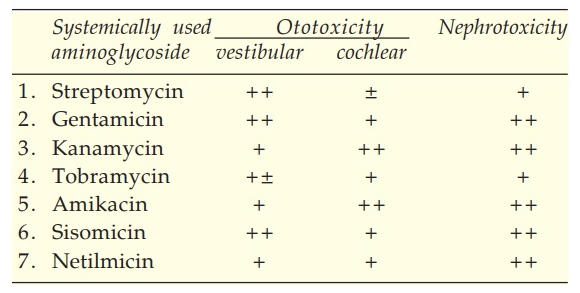
The antibiotics known as aminoglycosides are effective against both gram-positive and gram-negative bacteria. After oral treatment, these antibiotics are not absorbed, and nephro- and ototoxicities restrict their systemic usage. Ototoxicity’s effects could be especially severe. These antibiotics frequently harm the 8th cranial nerve’s auditory and vestibular branches. More frequently, especially with gentamicin and streptomycin, vestibular involvement is seen. Vertigo, nausea, and sometimes vomiting are possible symptoms; however, when medication is stopped, most patients fully recover. Hearing loss is irreversible when the auditory branch is damaged; auditory toxicity seems to be more likely with amikacin, kanamycin, neomycin, and netilmicin.
Mechanism of Pharmacologic Action
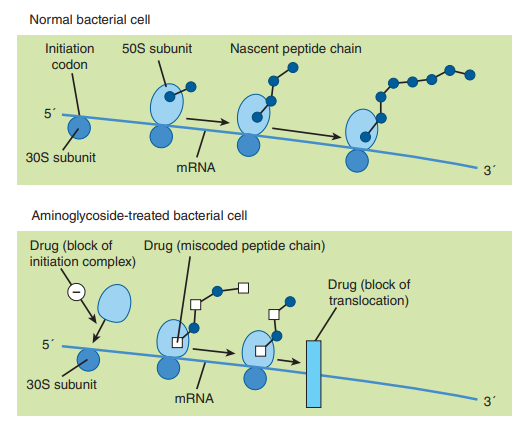
By interfering with the initiation complex between RNA and the 30S subunit or by obstructing translocation, aminoglycoside antibiotics operate on the 30S subunit of 70S ribosomal systems to cause particular misreading of the genetic codon and to prevent the synthesis of vital bacterial proteins. Nonetheless, it is thought that blocking protein synthesis is the more therapeutically significant mechanism of action. Proteins lacking the characteristic physiologic function of typical microbial proteins are produced as a result of the incorrect reading of coded information.
Mechanisms for Resistance
A growing issue with aminoglycoside antibiotics is the emergence of resistance strains, particularly among gram-negative bacilli, staphylococci, and mycobacteria. This has partly contributed to a decline in the therapeutic efficacy of drugs like kanamycin, neomycin, and streptomycin. Chromosome involvement (alteration of the 30S ribosomal subunit’s reactive site), plasmid transfer of extrachromosomal R-factors, and exclusion of the antibiotic from the bacterial cell are a few examples of known mechanisms of resistance. The main clinical issue is resistance brought on by R-factor transfer; acetylation, adenylation, or phosphorylation are the methods employed to enzymatically inactivate one or more aminoglycosides. Depending on the kind of metabolic inactivation involved, cross-resistance among the various aminoglycoside antibiotics is frequently complete, whereas no or just partial cross-resistance is reported with some bacteria. For example, if streptomycin is inactivated by phosphorylation of the 3-hydroxyl function of 2-deoxy-N-methylglucosamine, cross-resistance can be expected with kanamycin and paromomycin; if the same site adenylated, cross-resistance occurs with spectinomycin.
The therapeutic relevance of aminoglycoside antibiotics is influenced by the requirement for the therapeutic control of gram-negative and mycobacterial pathogens. However, in order to assure efficient use of these medications, more effort must be made due to the high frequency of resistance and the significant diversity that has been observed in some biologic features of the antibiotics. Blood levels should be periodically checked, and strain sensitivity should be regularly assessed. For the majority of aminoglycoside antibiotics and the majority of infections, a serum plasma level between 4 and 16 micrograms per milliliter is typically sought, and dosing regimens should be altered as necessary individually. Their excretion, which is primarily by glomerular filtration, is significantly impacted by renal diseases. Biologic half-lives can be extended by renal impairment from 2 or 3 hours to several days; in these circumstances, the dose regimen must be drastically altered to prevent prolonged high serum levels and the heightened risks of hazardous reactions that go along with them.
Only in cases where less toxic antibiotics are ineffective or inappropriate are aminoglycoside antibiotics recommended for the treatment of serious infectious diseases.
Streptomycin
The discovery of penicillin’s therapeutic potential sparked a vigorous quest for more antibiotic compounds. The identification of antibiotics hostile to gram-negative bacteria was a specific goal of these research. In 1944, Waksman and colleagues discovered streptomycin from a strain of Streptomyces griseus after observing the in vitro inhibitory impact of these species’ metabolites on gram-negative bacteria.
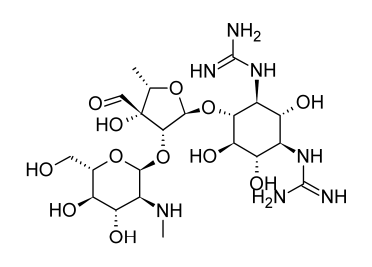
Studies into the origin and characteristics of the first aminoglycoside antibiotic identified, streptomycin, serve as the foundation for much of the present information about this class of antibiotics. Streptomycin is made up of streptidine and the disaccharide streptobiosamine, which has the sugar residues streptose and 2-deoxy-2-methylamino-L-glucose. All three of these components, according to biosynthetic research, are made from D-glucose. The connecting of the three components is not completely understood, but it is most likely the final stage of the biosynthetic process. The biosynthetic origins of the components of streptomycin can provide a general indication of the metabolic links of glucose to the various moieties despite the lack of detailed understanding on the production of individual moieties of aminoglycoside antibiotics.
The systemic use of streptomycin results in the nephro- and ototoxicities typical of the aminoglycoside medicines. Even on topical contact, the high prevalence of streptomycin hypersensitivity is not as significant. It is likely that hypersensitivity in this case is related to hapten production involving the formyl group of the streptose unit in streptomycin, even though hypersensitivity is not a common adverse reaction to aminoglycoside antibiotics as a whole.
Streptomycin is only explored for therapeutic use when adequate alternatives are unavailable due to the potential toxicity associated with systemic usage. Streptomycin is a first-choice antibiotic for treating tuberculosis, which is the main ailment needing systemic administration and is resistant to the majority of antibiotic therapies. To get the best results when treating tuberculosis, streptomycin is typically coupled with ethambutol and isoniazid. There is insufficient evidence to support earlier claims that streptomycin’s toxicity could be decreased by pairing it with dihydrostreptomycin, which can be made chemically from streptomycin or fermentatively with Streptomyces humidus by catalytic reduction of the formyl substituent on the streptose unit. It is now known that dihydrostreptomycin causes more serious auditory impairments than streptomycin does.
For best results, streptomycin should be used in combination with a sulfonamide to treat Yersinia pestis (the plague) and Francisella tularensis (tularemia). Sometimes the use of combined streptomycin-penicillin and streptomycin-tetracycline therapies is advised for treating bacterial endocarditis and brucellosis, respectively.
Neomycin and Paromomycin
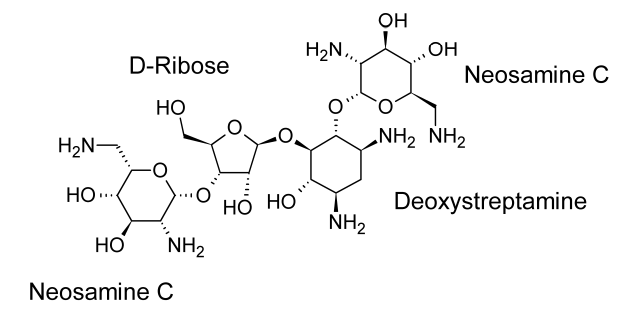
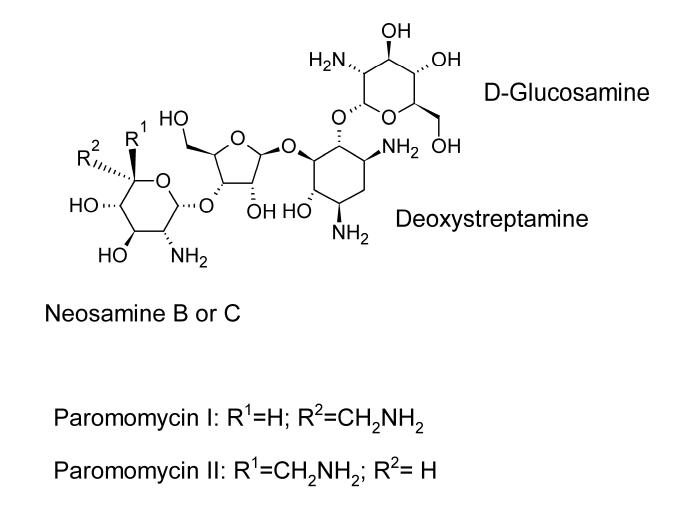
Neomycin and paromomycin are combinations of aminoglycoside antibiotics that were obtained from Streptomyces fradiae and Streptomyces rimosus var. paromomycinus, respectively, in 1949 and in 1959. The antibiotic molecules have three sugar residues and a 2-deoxystreptamine unit.
Neomycin is a concoction of three or more antibiotic substances. The major ingredient in the mixture is neomycin B. Just the stereochemistry of the aminomethyl group in the aminosugar connected to the ribose residue separates Neomycin C from Neomycin B. Only one sugar residue (neosamine C) is connected to the deoxystreptamine aglycone in neomycin A, also known as neamine.
At least two distinct antibiotics are present in paromomycin. These substances are analogues of neomycins B and C and have been given the names paromomycins I and II. The main component of the mixture is paromomycin I, and paromomycins differ from neomycins by having one amino group replaced with a hydroxyl functional group.
These antibiotics exhibit the spectrum of activity that is often associated with aminoglycoside antibiotics, are stable, and are not absorbed after oral administration. For the pre- or postoperative decrease of the intestinal flora, neomycin or other aminoglycoside antibiotics can be administered orally to control intestinal infections by susceptible organisms. These antibiotics function as an efficient supplementary therapy for hepatic coma because they lower the number of ammonia-forming bacteria in the digestive tract.
Kanamycin
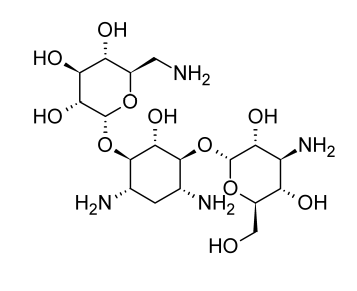
Kanamycin, a combination of at least 3 aminoglycoside antibiotics, was first discovered from Streptomyces kanamyceticus in 1957. These antibiotics have two distinct aminosugars connected to a 2-deoxy-streptamine aglycone. The majority of the mixture is made up of kanamycin A.
The activity spectrum of kanamycin is comparable to that of other antibiotics that are aminoglycosides. For preoperative care and infection management, it is taken orally. Kanamycin is effective against coliform bacteria, and Proteus species are typically more sensitive to it than to the more traditional aminoglycoside medicines. For sensitive gram-negative bacilli, the minimum inhibitory concentrations (MICs) typically range from 4 to 8 micrograms per milliliter. When susceptible germs are present, kanamycin can be administered parenterally for the treatment of severe gram-negative infections. Amikacin, gentamicin, and tobramycin have taken the place of kanamycin for systemic treatment of the majority of gram-negative infections due to the problem of emerging resistance.
Gentamicin
Actinomycete Micromonospora purpurea produces gentamicin. The main components of the antibiotic cocktail used in medicine are gentamicin C1, C1A, and C2. The majority of it (around 60%) consists of gentamicin C1. These antibiotic compounds have two aminosugar residues and one unit of 2-deoxystreptamine. Except for tobramycin, gentamicin inhibits pathogenic enterobacterial species such Enterobacter, Escherichia, and Klebsiella as well as Proteus and Serratia species at lower concentrations (typical MIC, 1 to 2 micrograms per milliliter). Moreover, it has a clinically significant effect on Pseudomonas aeruginosa (MIC 2 to 8 micrograms per milliliter); combined carbenicillin-gentamicin therapy may be particularly helpful in containing systemic Pseudomonas infections. Gentamicin is mostly used parenterally to treat severe gram-negative infections brought on by sensitive organisms, while it is also available in formulations (0.1%) for topical application.
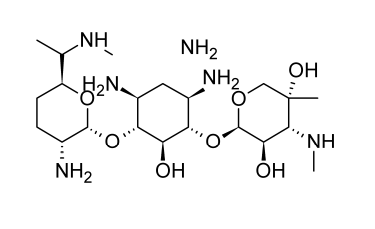
Cross-resistance with other aminoglycoside antibiotics seldom develops in clinical settings, but gentamicin resistance does exist; this absence of cross-resistance is likely due to R-factor-induced inactivation involving particular chemical sites that are not present in the gentamicin molecule (e.g., inactivation by adenylation or esterification of 3-hydroxyl function of a glucosamine moiety).
Tobramycin
The single-component antibiotic tobramycin, also known as nebramycin factor 6, is extracted from the nebramycin complex made by Streptomyces tenebrarius. This antibiotic compound is physically similar to kanamycin B and has two aminosugar residues and a 2-deoxystreptamine unit; the major difference is that the kanosamine residue lacks the 3-hydroxyl activity.
Midway through 1975, tobramycin received general medical approval. It has biochemical characteristics and clinical indications with gentamicin. Although Proteus vulgaris and Pseudomonas species are more sensitive to tobramycin in vitro and tobramycin may give somewhat lower tissue levels than gentamicin, these variations don’t seem to matter in clinical settings.
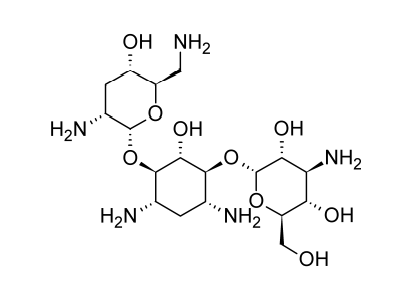
The sulfate salt of tobramycin is available, and the typical adult dose is 1 milligram of tobramycin per kilogram of body weight, either intramuscularly or intravenously three times each day.
Amikacin
Amikacin is a semi-synthetic aminoglycoside antibiotic that is made from kanamycin A by adding a L(-)-4-amino-2-hydroxybutyryl substituent to the 1-amino group of the deoxystreptamine moiety in an acylation mechanism. Amikacin is active against many types of pathogens that inactivate gentamicin, tobramycin, and other aminoglycoside antibiotics through enzymatic N-acetylation; the terminal amino group in this substituent is probably necessary for activity. Because amikacin-resistant pathogens are generally resistant to other known aminoglycoside antibiotics, some authorities advise cautious or restricted use of the drug.
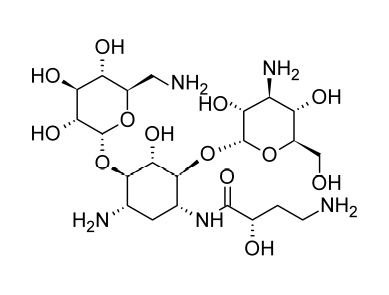
Amikacin is easily absorbed after being administered intramuscularly, and its typical serum half-life is 2 hours. The peak serum level shouldn’t be more than micrograms per milliliter, and the trough levels shouldn’t be more than 10 micrograms per milliliter, according to ototoxicity risks. To surpass the MICs of 90% of the strains of Escherichia coli, Enterobacter, Klebsiella, and Proteus, serum levels of 8 micrograms per milliliter are sufficient. 90% of the MICs for strains of Pseudomonas, Serratia, and Staphylococcus aureus must be reached at concentrations of 25% micrograms per milliliter.
Sisomicin
An antibiotic made from Micromonospora inyoensis is called sisomicin. Sisomicin has not been promoted in the United States despite being authorized for use in humans there. It has gentamicin-like antibacterial efficacy and efficiency against aminoglycoside-inactivating enzymes. Sisomicin displays pharmacological and pharmacokinetic characteristics that are comparable to those of gentamicin.
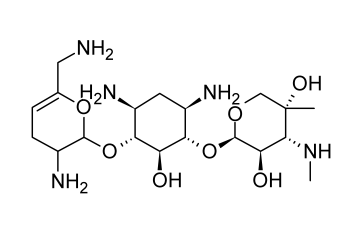
Netilmicin
Semi-synthetic aminoglycoside antibiotic netilmicin, also known as N-ethylsisomicin, is generated from sisomicin. Chemically, it is comparable to gentamicin C1A. Several gram-negative pathogens that are resistant to amikacin, gentamicin, and tobramycin can be killed off with it.
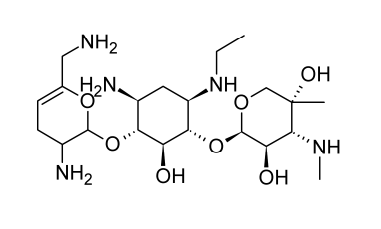
In the body’s tissues and organs, netilmicin is rapidly absorbed after parenteral delivery. The typical serum half-life lasts around two hours. According to ototoxicity concerns, the peak serum level shouldn’t be more than 16 micrograms per milliliter, and the trough shouldn’t be more than 4 micrograms per milliliter. Urine concentrations of up to 800 micrograms per milliliter are achieved, and nephrotoxicity appears to be less of a concern than with other aminoglycoside antibiotics. Other severe infections brought on by susceptible organisms as well as difficult urinary tract infections can benefit from its treatment.
Spectinomycin
Streptomyces spectabilis and Streptomyces flavopersicus generate the antibiotic spectinomycin. Though officially an aminoglycoside, the antibiotic molecule is a glycoside. A neutral deoxysugar is glycosidically bonded to an aminocyclitol aglycone. The dry antibiotic powder maintains its stability for a very long time.
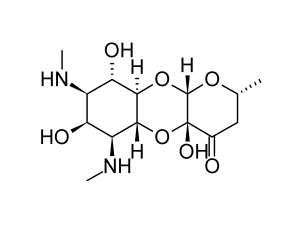
Several of the biologic characteristics of spectinomycin are comparable to those of the typical aminoglycosides. It is not absorbed when taken orally and is eliminated by glomerular filtration after injection in an active form. It inhibits protein synthesis by a mechanism involving the 30S subunit of the 70S ribosomal system.
Although it has a wide range of antibacterial effects, the only therapeutic use for the drug is the treatment of gonorrhea. A characteristic of this antibiotic that is exceptionally helpful for treating a venereal illness is that it typically controls susceptible strains of Neisseria gonorrhoeae (MIC range of 7.5 to 20 micrograms per milliliter). Many issues with social stigma and dispersed patient groups are eliminated as a result of this.
The use of spectinomycin should only be recommended in situations where penicillin is ineffective or inappropriate due to known spectinomycin resistance, according to some experts, who are also concerned about encouraging the establishment of more resistance. There is no known cross-resistance between penicillin and spectinomycin.
Engr. Dex Marco Tiu Guibelondo, BS Pharm, RPh, BS CpE
Editor-in-Chief, PharmaFEATURES
Print Media Acknowledgements
(1) Illustration of the Putative mechanisms of action of the aminoglycosides in bacteria. Katzung, B. (2018). Basic and Clinical Pharmacology, 14th Edition. Chapter 45: Aminoglycosides and Spectinomycin – Aminoglycosides: General Properties of Aminoglycosides (Mechanism of Action). p. 828. USA: McGraw-Hill Education. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(2) Structure of Streptomycin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Streptomycin Sulfate, Sterile). p. 297. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(3) Structure of Neomycin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Neomycin Sulfate). p. 298. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(4) Structure of Paromomycin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Paromomycin Sulfate). p. 298. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(5) Structure of Kanamycin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Kanamycin Sulfate). p. 299. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(6) Structure of Gentamicin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Gentamicin Sulfate). p. 300. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(7) Structure of Tobramycin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Tobramycin Sulfate). p. 300. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(8) Structure of Amikacin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Amikacin Sulfate). p. 299. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(9) Structure of Sisomicin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Sisomicin Sulfate). p. 301. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(10) Structure of Netilmicin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Netilmicin Sulfate, Sterile). p. 300. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
(11) Structure of Spectinomycin. Beale, J. Jr. & Block, J. (2011). Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry, 12th Edition. Chapter 8: Antibacterial Antibiotics – Aminoglycosides: Products (Spectinomycin Sulfate, Sterile). p. 301. USA, MD & PA: Lippincott Williams & Wilkins, a Wolters Kluwer business. The image was retrieved on February 23, 2023 via a digital copy of the aforementioned reference.
Subscribe
to get our
LATEST NEWS
Related Posts
Infectious Diseases & Vaccinology
Rezzayo™’s Latest EU Approval for Invasive Candidiasis Breaks Ground in Antifungal Therapy
Rezafungin marks the initial addition to the treatment arsenal for patients grappling with invasive candidiasis in more than 15 years.
Infectious Diseases & Vaccinology
Unmasking the Shadow: CDC Battles the Latest Fungal Meningitis Outbreak in Matamoros, Mexico
CDC tackles fatal fungal meningitis outbreak linked to surgeries in Matamoros, Mexico.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.