The race to cure sickle cell disease (SCD) is an exciting one, which is seeing pharma companies like Novartis and Bluebird Bio compete to bring their gene therapy to market first. The benefit of competition like this will contribute to continuous refinement of gene therapies for SCD, producing the most effective treatment with long-term benefits and a sufficient safety profile.
Introduction
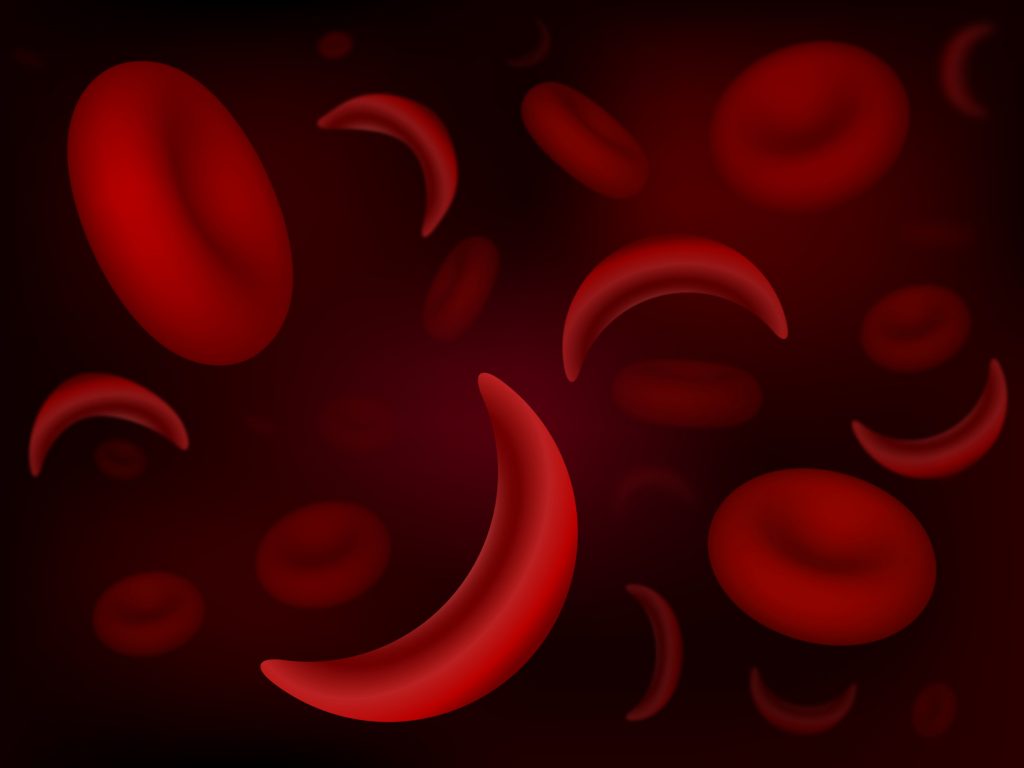
SCD is a group of rare, inherited blood disorders characterised by the presence of sickle or crescent-shaped red blood cells. The abnormal shape results in stiff and sticky cells which interact with other cells in the bloodstream and often causes clotting and blocking of the capillaries in the peripheral blood system.
As a result, this prevents healthy blood flow and delivery of oxygen/nutrients around the body, resulting in a number of health conditions. Examples include chest pain and pain of the bones which can be excruciating. The more serious conditions include stroke, eye problems, severe infections in children and episodic pain known as pain crises.
SCD represents an unmet clinical need due to the prevalence of the diseases, current treatment, and access to treatment in low-and-middle income countries. Approximately 5% of the global population carry the trait genes for hemoglobin disorders, mainly SCD and thalassaemia. 300,000 individuals are born every year with SCD which arises due to a mutation on chromosome 11.
Despite researchers understanding the genetic cause for SCD for many decades, only recently has research begun to develop experimental gene therapy that could offer effective treatment for thousands of people. Current treatment is generally focused on symptom management – so Ibuprofen for pain crises and dietary supplements.
The only cure so far is stem cell/bone marrow transplants. Unfortunately however, these are generally only considered in children with severe symptoms who do not respond to other treatment. In addition, there is an elevated risk due to graft versus host disease (GVH) and finding a suitable donor is a very difficult task.
The potential for CGT
A number of pharmaceutical companies have been researching SCD for many years and working towards treating the condition. Experimental gene therapies using the latest technologies appear to be the focus for a number of organisations, as the direction for treating SCD.
SCD has been considered a good candidate for gene therapy because a normal phenotype can be restored in pathological cells with a single copy of the mutated gene via a viral vector. If successful in clinical trials, this treatment could provide a viable cure for individuals living with SCD who would no longer rely on symptom management.
Other advantages include the elimination of long-term symptoms, allowing patients to avoid timely and costly blood transfusions. Gene-based treatments could also prevent one of the main causes for hospitalisation – vaso-occlusive crises – to which patients require strong painkillers which can increase the risk of addiction.
Novartis/Intellia, CRISPR/Vertex and Bluebird bio are a few of the pharma companies that are testing experimental gene therapy which are currently in clinical trials. Novartis is currently in phase 1/2 of its experimental gene therapy which is based on Intellia Therapeutics’ gene editing CRISPR technology.
The gene editing technology of CRISPR has shown to modify the DNA of stem cells via the deletion of a gene known as BCL11A, responsible for suppressing fetal hemoglobin production. As a result, the stem cells begin to produce this fetal hemoglobin which enables patients with congenital haemoglobin defects, e.g. SCD, to produce enough to overcome the effects of defective red blood cells.
Unlike other forms of gene therapy, CRISPR gene editing for SCD does not use a viral vector but via electroporation. In this method, controlled electric pulses produce temporary permeabilised areas in the cellular membrane This allows the transfer of molecules ranging from ions, to drugs to nucleic acids into target cells.
One of the main advantages is that unlike stem cell transplants, this technique uses the patient’s own cells, which avoids the risk of GVH disease and the lengthy wait for donors. In addition, this method removes the need for a biological vector and is known to have low risk off-target gene activation, which improves the safety profile.
Bluebird Bio’s approach
Bluebird Bio’s approach to SCD gene therapy is similar to Novartis, but does not utilise CRISPR technology, and is currently ahead in phase 3 clinical trials. LentiGlobin gene therapy is the investigational treatments being studied as a potential therapy for SCD as a part of BlueBird Bio’s clinical development programme.
The therapy works by delivering functional copies of a modified form of the beta-globin gene into the patients’ red blood cell precursors. Once these precursors differentiate, the red blood cells start producing the modified versions of haemoglobin, which, like Novartis’ therapy, should reduce the proportion of dysfunctional red blood cells that arise from the sickling form of haemoglobin.
Recently, Bluebird Bio’s gene therapy for SCD was granted an ‘innovation passport’ from the UK Medicines and Healthcare products Regulatory Agency (MHRA). This pathway forms a new UK approval process, which aims to reduce the time it takes for innovative therapies to reach the market. In a statement, Bluebird said that “if LentiGlobin is approved it could become the first one-time treatment for the rare blood disorder in the UK”.
The positive data from phase I and phase II has not been without complications however. The LentiGlobin program has experienced multiple setbacks with concerns surrounding patient safety, with reactions to the gene therapy. In February 2021, two of the SCD studies were halted after two participants developed leukemia-like cancer. On February 16, the company’s stock price plunged by 38%.
Since then however, an investigation was conducted by Bluebird Bio who released a statement saying that “it is very unlikely the suspected unexpected serious adverse reaction of acute myeloid leukemia reported in its Phase 1/2 study of LentiGlobin gene therapy for sickle cell disease was related to the BB305 lentiviral vector”.
After several halts in clinical trials, the LentiGlobin programme is running at full steam ahead and currently recruiting for phase 3. The latest data from the previous phases and the innovation passport put BlueBird Bio in a strong position for developing the first, effective gene therapy for SCD.
Charlotte Di Salvo, Lead Medical Writer
PharmaFeatures
Subscribe
to get our
LATEST NEWS
Related Posts

Featured
Sygnature Discovery Teams Up with Axol Bioscience to Unleash Human iPSCs’ Power
Sygnature Discovery partners with Axol Bioscience to explore hiPSC-derived microglia for antineurodegenerative drug discovery.
Cell & Gene Therapy
Telethon, Licensed to Leverage Takara Bio’s RetroNectin®
Takara Bio collaborates with Fondazione Telethon ETS in a RetroNectin® license agreement.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.