Veramed, a leading Specialist Biometrics CRO, has recently made headlines with its strategic move to acquire CTDS, a Boston-based data management, biostatistics, and medical writing company. This acquisition not only strengthens Veramed’s global presence but also expands its data management capabilities, solidifying its position as a key player in the biopharmaceutical industry.

Matthew Jones, Co-Founder and CEO of Veramed, envisions substantial growth with this amalgamation, emphasizing the synergy that will propel the combined team of over 350 statisticians, programmers, and data managers. Jones asserted that the benefits of the amalgamation would extend to the customers and staff of both organizations, opening doors to exciting opportunities with new partners. This strategic move aligns seamlessly with Veramed’s commitment to delivering end-to-end biometrics support, showcasing the company’s strategic foresight.
Biometrics Innovation: Navigating the Biotech Landscape
In the dynamic landscape of the biotech industry, specialized biometrics and data management providers like Veramed emerge as a pivotal solution. These providers offer specialized expertise in data collection and analysis, ensuring accurate results, maintaining regulatory compliance, and expediting decision-making processes. Collaborating with Veramed not only mitigates risks but also provides biotech companies access to advanced tools, fostering credibility with investors and facilitating effective research.
Founded in 2005 by Terri Sampo, CTDS has been a beacon of meticulous oversight and personalized service for small to midsize sponsor companies. The alignment of CTDS’s values with Veramed’s commitment to quality and delivery sets the stage for a seamless integration of their teams.
Veramed’s Evolutionary Services
Veramed’s commitment to staying at the forefront of industry evolution is evident in its array of services that span the biopharmaceutical lifecycle.
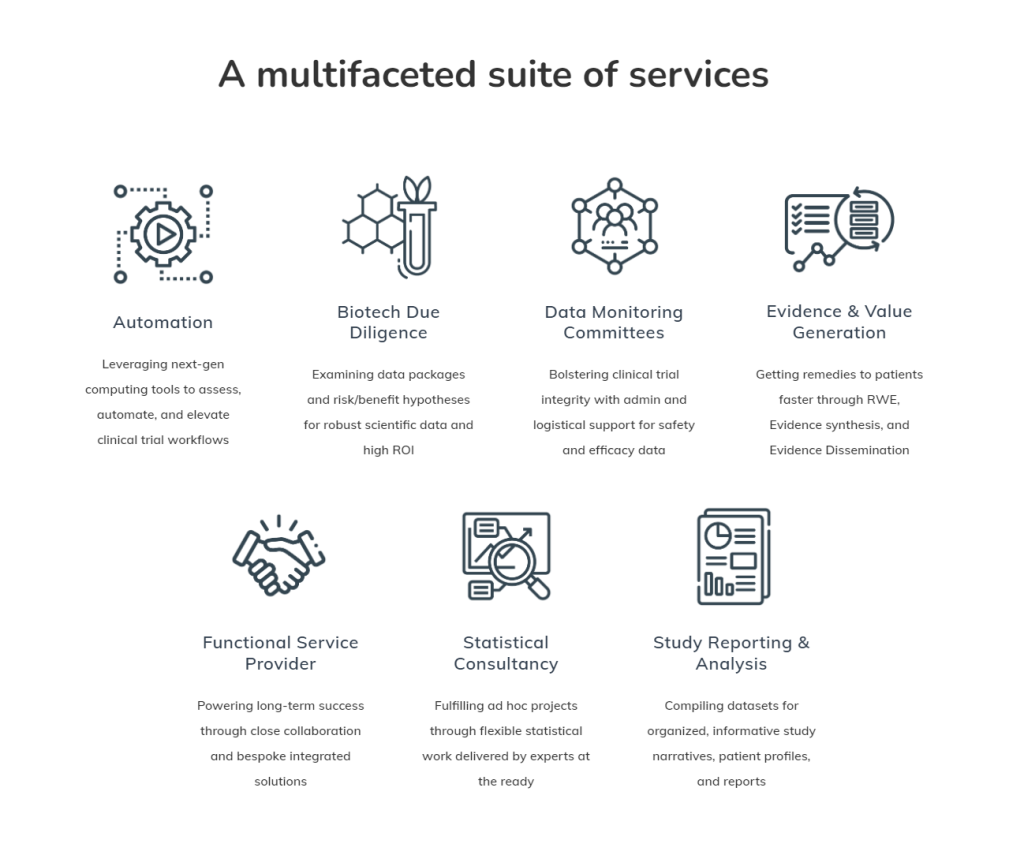
Automation: Revolutionizing Clinical Data Workflows
The clinical trial data landscape is evolving rapidly, and Veramed recognizes the need for adaptation. Their Automation, Efficiency, and Standards group offer consultancy on architectural processes, implementation of emerging automation tools like Domino Data Lab, and a dedication to open-source programming. By optimizing clinical data workflows, Veramed empowers pharmaceutical and biotech companies to scale, react, and deliver more effectively.
Veramed’s technical expertise encompasses high-level software design, Proof of Concept (POC) services, and consultancy on modern tools within the clinical data lifecycle, emphasizing open-source technology adoption and implementation.
Biotech Due Diligence: Decoding the Scientific Value
In the realm of external collaboration, Veramed’s Biotech Due Diligence services become a critical player. Their bespoke framework assesses the risk/benefit hypothesis, ensuring that scientific data aligns with investment decisions. Early pre-clinical, late pre-clinical, and late clinical stages are meticulously examined, delivering headline reports, detailed technical reports, and risk/benefit matrices.
Veramed’s due diligence process answers crucial questions about target relationships, exposure/response relationships, safety margins, and therapeutic hypotheses. The outcome is a comprehensive understanding of the asset’s scientific viability.
Data Monitoring Committee: Safeguarding Studies and Business Interests
Veramed’s Data Monitoring Committee (DMC) services not only focus on patient safety and study integrity but also bring invaluable benefits to the business. Acting as an independent entity, DMCs save time, money, and resources for sponsors, enhancing the overall efficiency of clinical trials.
The DMC services, underpinned by Veramed’s experience and expertise, encompass everything from setup to end-to-end Statistical Data Analysis Center support. This ensures seamless operation, timely access to relevant analyses, and effective oversight of unblinded results.
Evidence and Value Generation: Navigating Patient Data Dynamics
Veramed recognizes the wealth of patient data available and capitalizes on real-world evidence to design and execute high-quality studies. Whether it’s augmenting trials in rare diseases or validating patient-reported outcomes, Veramed’s Evidence and Value Generation services offer actionable insights.
Their evidence synthesis involves sophisticated statistical models, supporting regulatory submissions and payer-facing interactions. With a focus on data visualizations, medical affairs support, and strategic publication planning, Veramed ensures that scientific insights reach key stakeholders effectively.
Functional Service Provider (FSP): A Framework for Success
Veramed’s Functional Service Provider (FSP) model provides a strong framework for collaboration. Focused on open communication, long-term resource assignment, and senior accountability, Veramed ensures that partnerships deliver quality support within timelines and budget.
Key factors for success include maintaining a commitment to quality, long-term resource engagement, and strong technical knowledge. Veramed’s approach as an extension of the client’s team fosters successful, value-driven partnerships.
Statistical Consultancy: Flexible Support for Robust Trials
Veramed’s Statistical Consultancy services offer flexibility and collaboration throughout the clinical trial journey. From clinical study design and protocol development to simulations and randomization, Veramed’s statisticians provide support tailored to the client’s needs.
Whether in setup activities, midstream processes, analysis, and reporting, or post-reporting activities, Veramed’s statistical consultancy adapts to fluctuating demands, ensuring a robust statistical foundation for clinical trials.
Study Reporting & Analysis: A Focus on Quality
Veramed’s Study Reporting & Analysis services tie together its biometrics expertise, offering tailored solutions for clients. With a meticulous Quality Control process, Veramed’s programing team ensures the accuracy of datasets and the statistical integrity of clinical study reports.
Whether it’s protocol reviews, vendor oversight, analysis dataset development, or CDISC documentation, Veramed’s comprehensive approach to study reporting guarantees submission-ready deliverables. Their expertise in CDISC SDTM conversion and ADaM implementation solidifies their commitment to maintaining industry standards.
The Veramed Vision for Biometrics Excellence
Veramed’s strategic leap through the acquisition of CTDS and its diverse suite of biometrics services reflect a commitment to innovation, quality, and adaptability. By seamlessly integrating expertise across the biopharmaceutical lifecycle, Veramed stands as a pivotal partner for pharmaceutical and biotech endeavors. The emphasis on evidence generation, functional service provision, statistical consultancy, and study reporting positions Veramed at the forefront of the evolving biometrics landscape, poised to drive success and excellence in the industry.
About Veramed
Veramed, a pioneering clinical research organization headquartered in London, stands at the forefront of redefining modern biometrics in the pharmaceutical landscape. As a certified B Corp, they prioritize people-centric approaches and extend their services globally, with offices in the UK, US, EU, and Ukraine, ensuring close collaboration across mid-stream, analysis and reporting, as well as post-reporting & submission stages of the clinical trial process.
Founded in 2012 by a statistician and a programmer who had experienced subpar service quality and poor work-life balance in their previous roles within the healthcare industry, Veramed embarked on a mission to set higher standards both as a solution provider and an employer. Uniquely practitioner-led, the company fosters a fulfilling workplace culture that values work-life balance, positioning Statisticians and Programmers as integral members of the Veramed team.
Over more than a decade, Veramed has organically grown to establish a global presence, offering specialized services to pharma and biotech companies worldwide, regardless of in-house expertise. The company prides itself on challenging industry norms and doing things differently, striving to showcase this difference every day.
Veramed’s mission is to bring people, innovation, and technology together with world-class governance to accelerate evidence generation and advance patient health. Their vision is to be a pioneering CRO that redefines intelligent healthcare decision-making through end-to-end biometrics.

The core values of Veramed, woven into the fabric of its culture, revolve around three pillars of quality: Integrity, Collaboration, and Excellence. The commitment to honesty and transparency, collaboration with diverse talents, and the pursuit of a higher standard in services and responsibilities underline their dedication to quality.
Recognized as a world-class three-star employer by Best Companies, Veramed not only maintains a positive culture and work-life balance but also invests in employee training, development, and support programs. The Veramed Excellence Program, regular social events, mental health awareness initiatives, and a commitment to a fulfilling team environment demonstrate their ongoing efforts to offer the best workplace for their staff.
Veramed’s role as a high-performing partner in drug development is acknowledged through the Best Contract Research Organization – Specialist Providers award at the 18th Annual Scrip Awards in 2022. This recognition highlights their significant contributions to the industry and their dedication to making a difference in patient communities worldwide. Veramed’s passion for challenging industry norms and staying innovative reflects their commitment to shaping the future of healthcare through their work and collaborations.

More about Veramed from this link.
About CTDS
Clinical Trial Data Services (CTDS), a woman-owned company, specializes in providing comprehensive data management, EDC database design, and biostatistical and medical writing services to pharmaceutical and medical device companies. Comprising a team of experienced senior professionals, CTDS adopts a consultative approach, fostering partnerships with clients to ensure well-run clinical trials and the production of valuable data.

Founded in 2005, CTDS has been instrumental in aiding small to mid-size sponsor companies in advancing their drug or device research and development. Their involvement spans a spectrum of clinical trials, from early-stage (phase I) studies to late-stage (phase III) and post-marketing studies, many of which have achieved regulatory approval. CTDS prides itself on delivering quality data that withstands regulatory scrutiny, supporting numerous regulatory applications.
The mission of CTDS revolves around providing high-quality consulting services to the pharmaceutical and medical device industries. Their collaborative efforts with sponsor companies aim to secure product approval, with an emphasis on integrating quality into every step of the process to produce accurate data for submission. The ultimate goal is to expedite the approval and market entry of promising new technologies, ensuring patients benefit from innovations sooner.
CTDS distinguishes itself through personalized service and customized solutions tailored to the unique needs of each project. Operating as an extension of clients’ teams, they remain responsive to mid-study changes. A transparent pricing model minimizes surprises and change orders. Leveraging their skills and experience, CTDS is committed to accelerating time to market, achieving database go-live within four weeks of final CRFs in 90% of their studies. Database lock typically occurs within three weeks of the last patient’s last visit, with topline results submitted within one week thereafter.
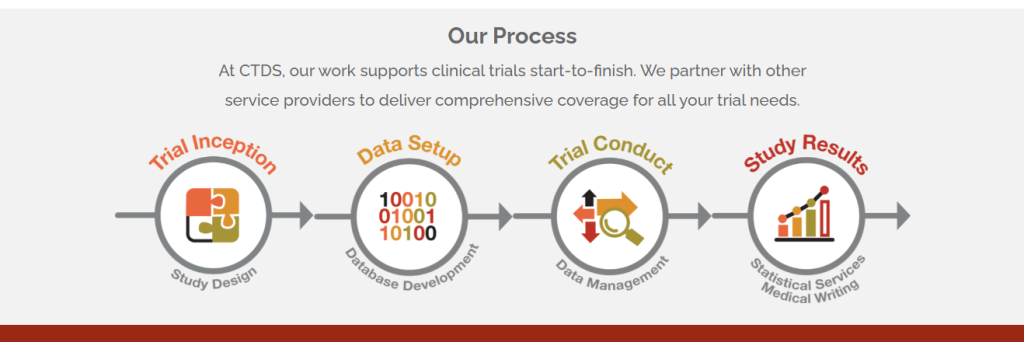
Accuracy is paramount for CTDS, with a dedicated focus on data quality and the application of rigorous standards to every trial. Meticulous oversight, attention to detail, and a steadfast commitment to process enable them to consistently deliver quality data, facilitating accurate analyses and reliable results.
Key statistics for CTDS underscore their track record of success: in business since 2005, an average employee tenure of 5 years, 85% customer retention, hundreds of studies performed, numerous FDA approvals, and notably, no FDA 483 warning letters. This cumulative experience and commitment to excellence position CTDS as a trusted and reliable partner in the field of clinical trial data services.
Learn more about CTDS from its Official Website.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

AI, Data & Technology
The Power of Unsupervised Learning in Healthcare
In healthcare’s dynamic landscape, the pursuit of deeper insights and precision interventions is paramount, where unsupervised learning emerges as a potent tool for revealing hidden data structures.

AI, Data & Technology
Unlocking Intelligence: A Journey through Machine Learning
ML stands as a cornerstone of technological advancement, permeating various facets of our daily lives.
Read More Articles
Synthetic Chemistry’s Potential in Deciphering Antimicrobial Peptides
The saga of antimicrobial peptides unfolds as a testament to scientific ingenuity and therapeutic resilience.