In the intricate world of medicinal chemistry and drug discovery, radiolabeled compounds have emerged as indispensable tools, offering a unique glimpse into the dynamic interplay of biological processes within living systems. These compounds, aptly named radioligands or radiopharmaceuticals, are becoming increasingly pivotal in unraveling the mysteries of pharmacology. Among the trailblazers in this field stands Advanced Innovative Partners (AIP), a Florida-based contract research organization, championing the use of state-of-the-art radiolabeling techniques to empower pharmaceutical companies in their pursuit of groundbreaking therapeutics.
Pushing the Boundaries of Medicinal Chemistry with Radioligands
Radioligands, through their radioactive signatures, facilitate the visualization and quantification of intricate biological processes, providing researchers with unprecedented insights into the behavior of drug candidates and molecular targets. Dr. Jane Doe, Head of Radioligand Sciences at AIP, emphasizes the unique advantage radioligands offer: “Radioligands give our chemist and biologist partners a unique window into the behavior of their compounds and targets in vivo that they simply can’t get through other techniques.”
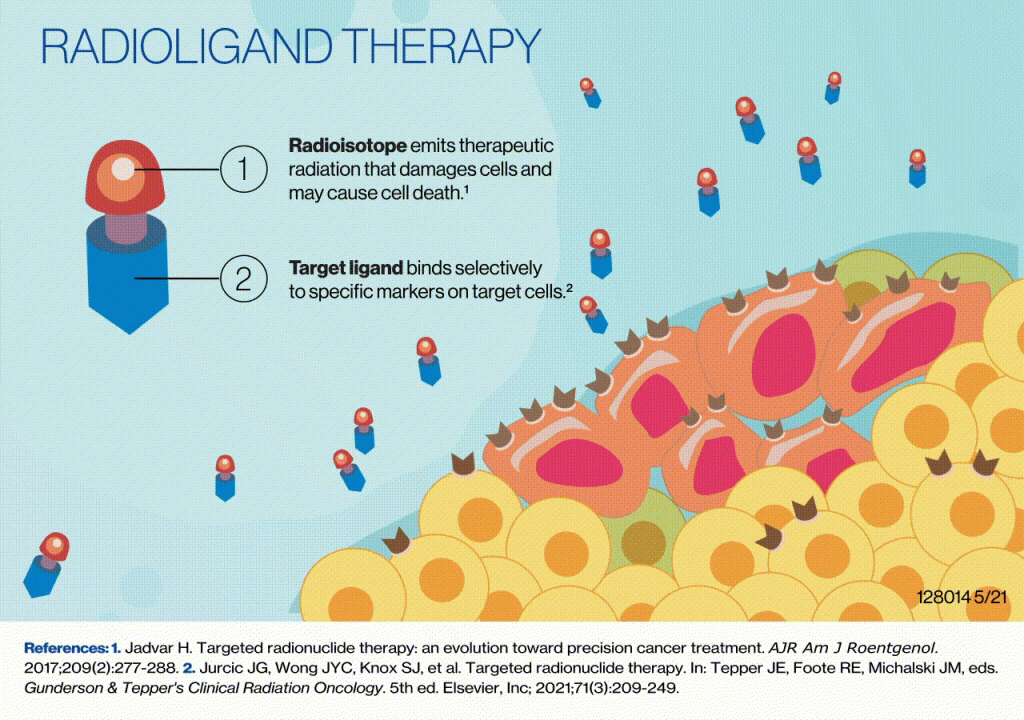
She adds that “by synthesizing and testing radiolabeled versions of lead compounds, we can provide crucial data on pharmacokinetics, target engagement, and drug occupancy.” This wealth of information empowers medicinal chemists to swiftly iterate on chemical structures, optimizing key properties such as potency and selectivity. The ability to identify off-target interactions also positions radioligands as indispensable in mitigating potential adverse effects.
Navigating the Molecular Landscape: AIP’s Radiolabeling Expertise
AIP’s prowess extends across a diverse range of molecular modalities and radionuclides. For small molecule programs, carbon-14 and tritium radiolabeling take the spotlight, allowing detailed mapping of the absorption, distribution, metabolism, and excretion (ADME) of compounds. This meticulous understanding of a compound’s journey within the body lays the foundation for informed drug design.
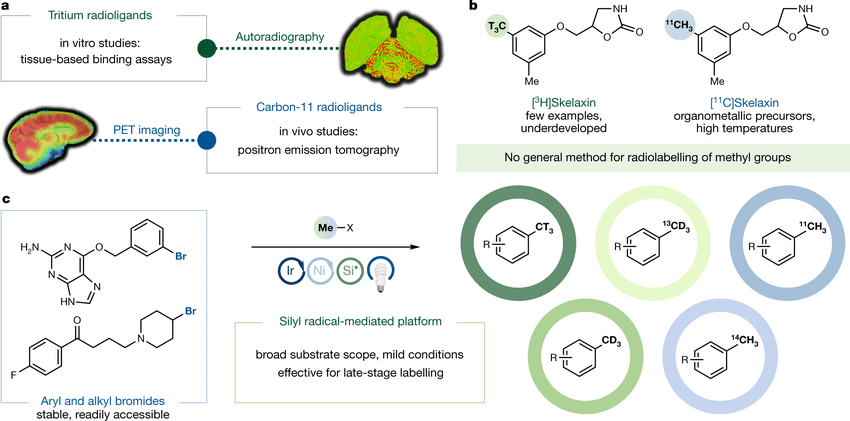
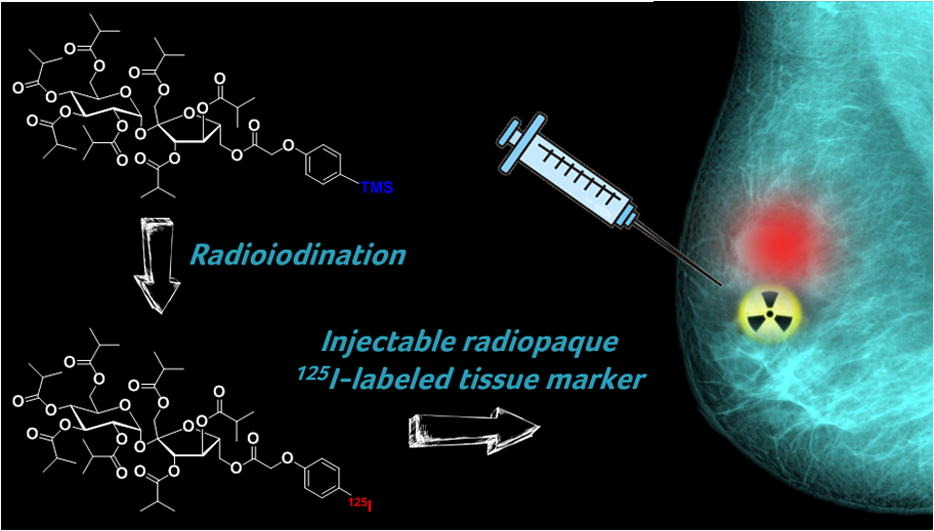
In the realm of larger biologics, AIP employs iodine-125 labeling, enabling the pharmacokinetic characterization of these complex molecules. Additionally, the team, under the leadership of AIP’s Co-Founder, Chairman and Chief Scientific Officer, Dr. Stanley Satz, specializes in the synthesis of positron emission tomography (PET) radiotracers using isotopes like carbon-11 and fluorine-18. These radiotracers facilitate non-invasive visualization of drug occupancy in the human brain, propelling advancements in central nervous system drug development.
AIP’s Commitment to Excellence and Innovation
Dr. Satz underscores AIP’s commitment to providing partners with the highest quality radioligand data, facilitating informed decisions and propelling the development of groundbreaking therapies. The organization’s relentless pursuit of excellence is evident in its continual expansion of capabilities to handle novel target classes, ensuring it meets the evolving needs of its clients. With its breadth of radiochemistry expertise and cutting-edge facilities, AIP is undeniably at the forefront of unlocking the full potential of radioligands, thereby charting the course for precision drug discovery and development.
About Advanced Innovative Partners (AIP)
Advanced Innovative Partners (AIP) emerges as a prominent global clinical-stage biotechnology company, standing at the forefront of diagnostic and therapeutic advancements in the realm of targeted radiation. Founded in 2017, AIP boasts a skilled team with expertise spanning biotechnology, nuclear medicine, and molecular biology. Their commitment lies in the development of next-generation diagnostic and therapeutic radiopharmaceuticals, addressing critical needs in oncology, rare pediatric diseases, infectious diseases, and biomedical countermeasures.

At the heart of AIP’s operations is a robust pipeline, extending its reach to breast, lung, brain, and solid tumor cancers, as well as rare diseases. AIP navigates this expansive landscape with a reliable, secure global supply chain and a collaborative network, ensuring the seamless progression of their innovative products from development to delivery.
With lead programs advancing through Phase I/II/III clinical trials, AIP positions itself as a leader in the emerging market space of radiopharmaceutical diagnostics and therapeutics. The company’s success is underpinned by patented platform technologies that underscore their commitment to excellence in treatment and clinical management. AIP’s approach is product-centric, reflected in the creation of a pipeline comprising multiple drugs strategically targeting areas with significant market opportunities.
AIP’s mission revolves around expediting the discovery, development, and global availability of transformative therapies. Their focus extends beyond innovation, emphasizing the importance of rigorous clinical trials mandated by regulatory bodies. By navigating these regulatory pathways and obtaining approvals, AIP aims to make their investigational products accessible to patients worldwide.
Central to AIP’s identity is a dedication to pioneering targeted therapies and advancing disease detection and treatment. This commitment unfolds through a comprehensive, integrated approach encompassing proprietary tech platforms, strategic partnerships, and an unwavering patient-centric focus. AIP’s ultimate goal is to profoundly impact patient journeys by enabling accurate diagnoses at an earlier stage and providing treatments that are not only more powerful but also gentler, thereby offering patients the best chance for improved outcomes. In essence, AIP aspires to make a meaningful difference for individuals grappling with complex, aggressive, rare, and infectious diseases worldwide.

Learn more about AIP from their Official Website.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Invisible Couriers: How Lab-on-Chip Technologies Are Rewriting the Future of Disease Diagnosis
The shift from benchtop Western blots to on-chip, real-time protein detection represents more than just technical progress—it is a shift in epistemology.

Medicinal Chemistry & Pharmacology
Designing Better Sugar Stoppers: Engineering Selective α-Glucosidase Inhibitors via Fragment-Based Dynamic Chemistry
One of the most pressing challenges in anti-diabetic therapy is reducing the unpleasant and often debilitating gastrointestinal side effects that accompany α-amylase inhibition.

Medicinal Chemistry & Pharmacology
Into the Genomic Unknown: The Hunt for Drug Targets in the Human Proteome’s Blind Spots
The proteomic darkness is not empty. It is rich with uncharacterized function, latent therapeutic potential, and untapped biological narratives.

Medicinal Chemistry & Pharmacology
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.











