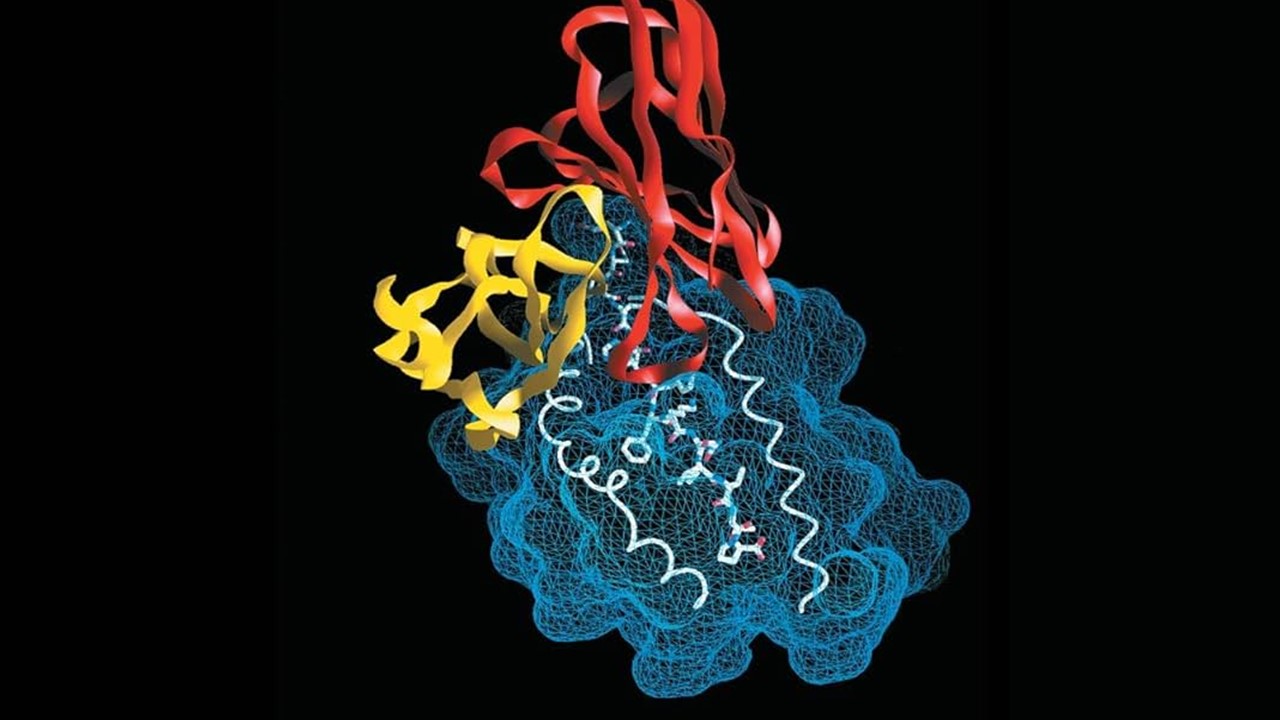
Alcohol use disorders (AUDs) represent a multifaceted challenge, with diverse drinking behaviors that vary significantly across individuals. Some people engage in daily drinking, others reserve it for weekends, while others consume alcohol in episodic binges. Despite these varied patterns, all forms of problematic alcohol use share a potential link rooted in genetics and molecular biology, prompting questions about how these factors shape behaviors and susceptibilities. In an innovative study, researchers sought to unravel this genetic landscape by integrating proteomic (protein) and transcriptomic (gene expression) data, revealing key proteins and genes associated with AUD. This systematic approach, combined with neuroimaging, has paved the way for identifying promising targets for therapeutic intervention.
Pharmacologic Overview of Ethanol: Targeted Receptor Interactions
Ethanol, the active component in alcoholic beverages, exerts its effects through complex interactions with various neurotransmitter receptors in the central nervous system. Primarily, ethanol acts as a positive allosteric modulator of the GABAA receptor, enhancing the inhibitory neurotransmission mediated by gamma-aminobutyric acid (GABA). This modulation leads to the sedative and anxiolytic effects commonly associated with alcohol consumption. Notably, ethanol’s potentiation is more pronounced in GABAA receptors containing the δ-subunit, which are predominantly located extrasynaptically and contribute to tonic inhibition. This specificity underscores the nuanced ways in which ethanol influences neural activity.
Beyond its interaction with GABAA receptors, ethanol also affects excitatory neurotransmission by acting as a negative allosteric modulator of NMDA-type glutamate receptors. This inhibition reduces glutamatergic activity, contributing to the cognitive impairments and memory disruptions often observed during intoxication. Additionally, ethanol modulates other receptor systems, including nicotinic acetylcholine receptors, serotonin 5-HT3 receptors, and glycine receptors, each contributing to the diverse physiological and behavioral effects of alcohol. The combined modulation of these receptor systems by ethanol results in the characteristic depressant effects on the central nervous system, highlighting the intricate pharmacological profile of this widely consumed substance.
Breaking Down the Four Dimensions of Alcohol Use Disorders
To precisely understand the biology behind alcohol use behaviors, the study zeroed in on four dimensions of AUD: the total number of drinks consumed per week, the prevalence of binge drinking, behaviors categorized as problematic drinking, and the frequency of alcohol intake. By classifying AUD in this way, the researchers sought to capture the nuances of drinking behaviors and link them to specific biological markers. Analyzing each dimension as a unique behavioral phenotype enabled the researchers to dig deeper into how genes and proteins might independently or collectively influence each behavior.
The four categories reflect distinct patterns of alcohol interaction, each with its own underlying biological and behavioral mechanisms. By mapping these categories, researchers have established a foundation for associating distinct genetic and proteomic signatures with varied drinking behaviors, laying the groundwork for personalized interventions and targeted treatment options. This nuanced classification system reinforces the idea that not all forms of AUD are driven by the same molecular mechanisms, even if they lead to similar consequences.
Proteomic and Transcriptomic Analysis: The Search for Molecular Markers
The study utilized an extensive dataset encompassing nearly 3,500 cortical proteins and 6,100 genes from canonical brain cell types. By assessing this massive volume of data, researchers identified 217 cortical proteins and 255 cell-type genes associated with AUD. Among these, 36 proteins and 37 genes had never before been associated with alcohol-related behaviors. The comprehensive identification of these molecular targets is a leap forward in understanding the cellular and biochemical underpinnings of AUD.
Interestingly, despite the extensive overlap in proteins and genes analyzed, the study found minimal intersection between proteomic and transcriptomic targets. This limited overlap suggests that AUD’s complexity might stem from distinct molecular pathways operating independently within various brain cell types. Such findings emphasize the need for a multi-layered approach to understanding AUD, as different forms of the disorder may be mediated by separate cellular mechanisms. This distinction is crucial in advancing future research efforts that aim to delineate specific molecular targets based on unique behavioral phenotypes.
Neuroimaging Reveals Shared Pathways in Alcohol Use Behaviors
To complement their molecular analyses, researchers employed neuroimaging techniques to explore downstream neurophysiological pathways associated with the identified genes and proteins. Neuroimaging provided a window into how molecular differences manifest in the brain, linking genetic variations to structural and functional changes. Shared pathways in neurophysiology indicate that certain brain regions or circuits may play a universal role in AUD, regardless of the type of problematic drinking behavior.
This integration of neuroimaging adds a critical dimension to understanding AUD, illustrating that while molecular targets may differ, they converge on shared neural pathways. These pathways could offer therapeutic targets for pharmacological interventions. For example, if specific brain regions are consistently affected by distinct genes or proteins linked to AUD, targeting these areas with medications could mitigate a range of alcohol-related behaviors. Thus, neuroimaging not only validates the molecular findings but also provides actionable insights into the biological mechanisms underpinning AUD.
Prioritizing Key Molecular Players: CAB39L, NRBP1, and the mTOR Signaling Pathway
Among the proteins and genes identified, the study highlighted 16 proteins and 12 cell-type genes with particularly strong associations with AUD, including CAB39L and NRBP1. These molecules play roles in cellular processes that might directly influence AUD susceptibility, providing potential targets for therapeutic development. CAB39L, for instance, is implicated in regulating cellular growth and metabolism, processes that may intersect with pathways involved in reward and addiction.
The mTOR signaling pathway, often associated with cell growth and survival, was also implicated. Known for its role in cellular energy management and synaptic plasticity, mTOR signaling has been previously linked to addictive behaviors and reward-related mechanisms in the brain. The presence of mTOR-associated proteins in AUD research suggests that manipulating this pathway could alter neural circuits related to alcohol consumption. Consequently, these findings underscore the relevance of pathways that might offer therapeutic options for modulating alcohol use behaviors.
Neuropsychiatric Implications: The Role of Genes with Favorable Profiles
In addition to the proteins and pathways linked directly to AUD, the study identified genes with neuropsychiatric profiles favorable for therapeutic targeting. Genes such as SAMHD1, VIPAS39, NUP160, and INO80E surfaced as notable candidates, with each playing unique roles in neural regulation. For instance, SAMHD1 is involved in nucleotide metabolism and cellular stress responses, which might impact brain resilience and vulnerability to substances like alcohol.
The implications of these genes extend beyond AUD; their roles in neuropsychiatric health suggest they could be targeted to improve mental health outcomes for individuals with coexisting psychiatric conditions. By focusing on genes that have broad neuropsychiatric profiles, researchers could develop treatments that address AUD while simultaneously improving overall brain health, a critical consideration given the frequent co-occurrence of AUD with disorders like anxiety and depression.
The Future of AUD Research and Treatment
The findings of this study underscore the vast potential for advancing precision medicine in treating AUD. The complexity of AUD’s genetic architecture, as revealed by proteomic and transcriptomic integration, suggests that a one-size-fits-all treatment approach may be inadequate. Instead, future therapies might focus on tailored interventions that consider individual genetic and proteomic profiles, offering personalized treatments that target specific molecular pathways associated with each individual’s unique form of AUD.
By identifying the distinct molecular markers and neural pathways involved in problematic drinking behaviors, researchers have laid the groundwork for developing drugs or therapies that precisely target these markers. Such advancements hold promise not only for treating AUD but also for improving our understanding of addiction as a whole. Precision medicine offers a beacon of hope, shifting the focus from generalized treatments to therapies that address the unique biological needs of each individual.
A New Paradigm for Understanding Alcohol Use Disorders
This study represents a significant step toward unraveling the complex molecular underpinnings of AUD. By integrating large-scale proteomic and transcriptomic data, researchers have provided new insights into the genetic factors that contribute to problematic drinking behaviors. Their work highlights both the diversity of molecular pathways involved in AUD and the potential for therapeutic targets that might one day offer relief to those struggling with alcohol-related issues.
As our understanding of AUD’s genetic and molecular landscape deepens, so does the potential for innovative treatments that go beyond traditional approaches. This research serves as a foundational step in recognizing and treating AUD not as a single disorder but as a spectrum of behaviors rooted in distinct biological pathways. The journey toward effective, targeted treatments continues, offering hope that, in the future, individuals will have access to therapies tailored to their unique genetic and proteomic profiles, transforming the way we understand and treat alcohol use disorders.
Study DOI: https://doi.org/10.1038/s41562-024-02040-1
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES
Today is Proventa International’s Clinical Operations and Clinical Trials Supply Chain Strategy Meeting at Le Meridien Boston Cambridge, Massachusetts, USA. Biotech thought-leaders and pharma industry titans will be engaging with like-minded delegate peers on the latest developments in the clinical space and regulatory affairs.

Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.
Medicinal Chemistry & Pharmacology
Coprocessed for Compression: Reengineering Metformin Hydrochloride with Hydroxypropyl Cellulose via Coprecipitation for Direct Compression Enhancement
In manufacturing, minimizing granulation lines, drying tunnels, and multiple milling stages reduces equipment costs, process footprint, and energy consumption.

Medicinal Chemistry & Pharmacology
Decoding Molecular Libraries: Error-Resilient Sequencing Analysis and Multidimensional Pattern Recognition
tagFinder exemplifies the convergence of computational innovation and chemical biology, offering a robust framework to navigate the complexities of DNA-encoded science