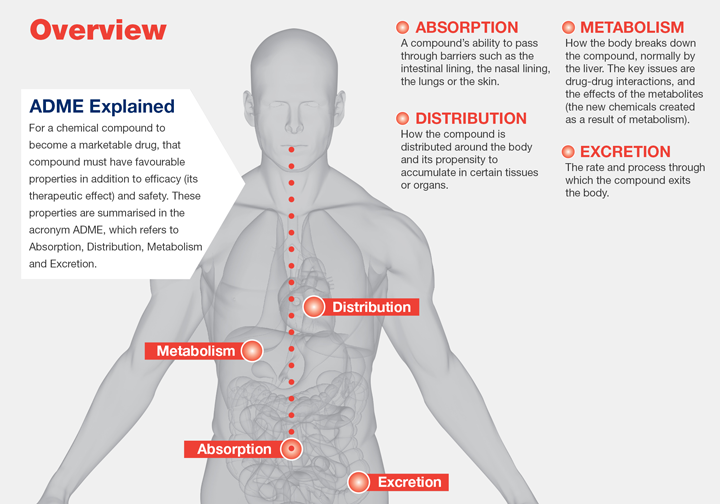
Bioavailability is a fundamental concept in pharmacology that delves into the intricate dynamics of drug absorption and distribution within the body. It encapsulates the extent and rate at which a drug reaches its intended site of action or systemic circulation. This critical measure plays a pivotal role in determining the efficacy and safety of pharmacotherapeutic interventions.
Impact of Route of Administration and Dosage
The route of administration (ROA) and dosage of a drug wield significant influence over its bioavailability. A delicate balance exists wherein the dose of a drug inversely correlates with its bioavailability. Altering the ROA necessitates adjustments in dosage to achieve optimal therapeutic outcomes. For instance, oral administration subjects drugs to gastrointestinal absorption and hepatic first-pass metabolism, potentially altering their bioavailability. In contrast, intravenous administration bypasses these processes, ensuring immediate delivery to systemic circulation and maximizing bioavailability.
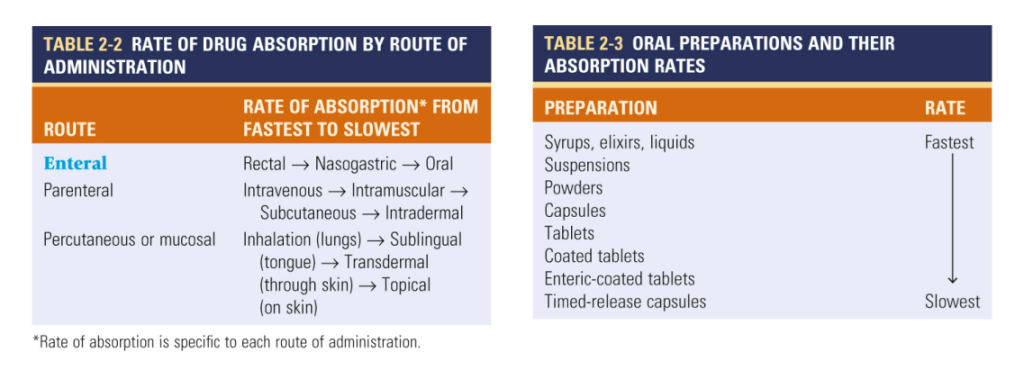
What is Drug Clearance?
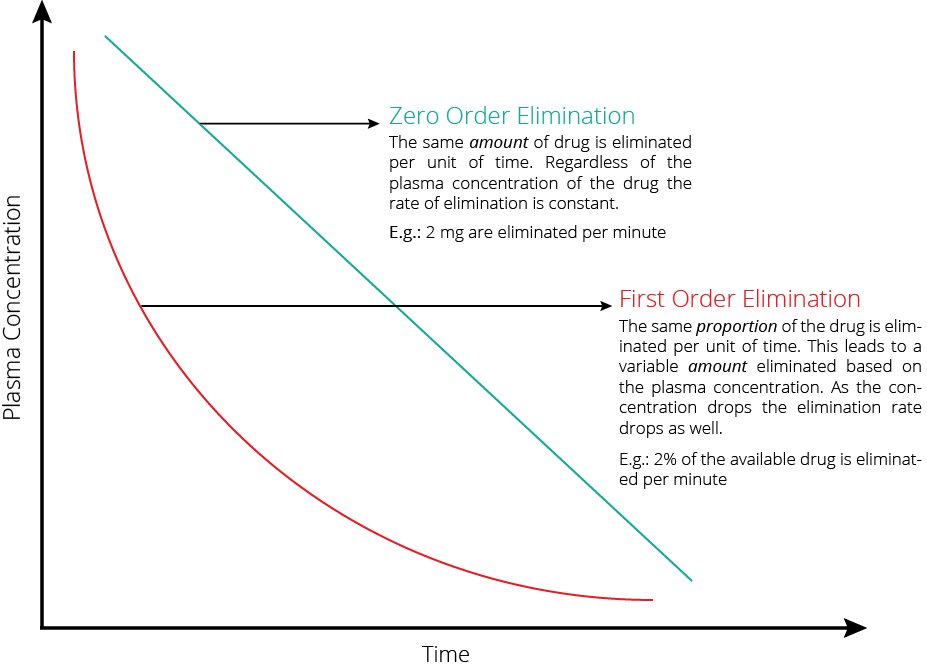
Drug clearance, the process by which active drugs are removed from systemic circulation, is a critical determinant of bioavailability. The distinction between first-order and zero-order kinetics guides our understanding of drug elimination dynamics. While first-order kinetics entail exponential elimination proportional to plasma concentration, zero-order kinetics denote constant drug elimination over time. Awareness of a drug’s clearance mechanism is paramount, as it directly impacts bioavailability and steady-state concentration, crucial considerations in therapeutic efficacy assessment.
Exploring Distribution Dynamics
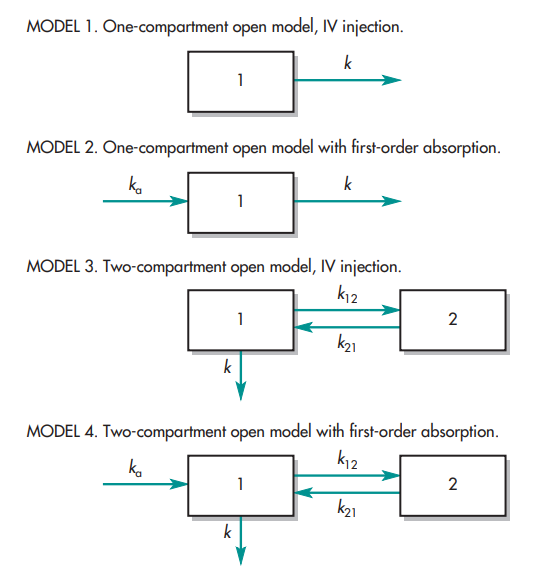
Distribution, the dispersion of drugs across various bodily compartments, illuminates the complexity of bioavailability. Whether in a single-compartment or multi-compartment model, the volume of distribution (Vd) serves as a key indicator of a drug’s distribution profile. A larger Vd implies extensive distribution beyond the central compartment, potentially influencing bioavailability. Understanding these distribution dynamics is instrumental in optimizing drug delivery strategies to maximize therapeutic efficacy.
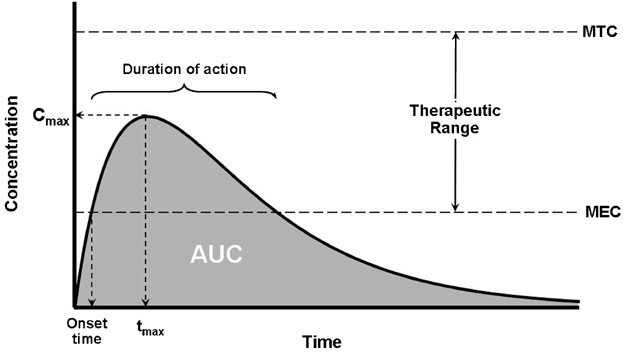
Quantifying Bioavailability
Bioavailability, often quantified through area under the curve (AUC) analysis, provides invaluable insights into drug absorption kinetics. By comparing AUCs of different administration routes, one can delineate bioavailability with precision. However, inherent limitations exist, particularly concerning assumptions of constant clearance and uniform drug distribution within plasma.
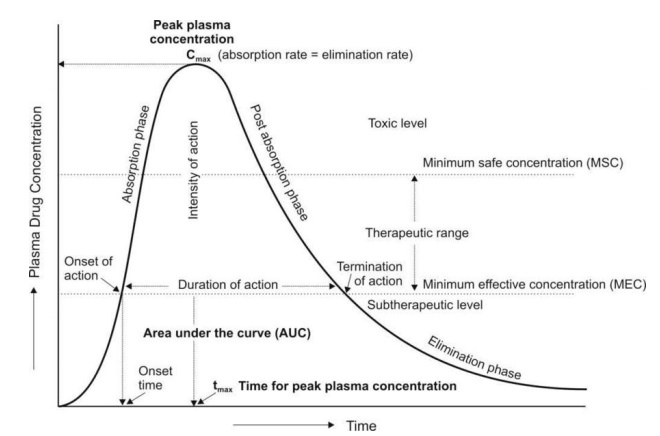
Navigating Clinical Relevance
In clinical practice, appreciation of bioavailability nuances is indispensable for informed decision-making. Interactions with intrinsic and extrinsic variables, such as metabolic pathways and concurrent medications, can significantly alter bioavailability. These considerations are exemplified in scenarios involving drugs like warfarin and nitroglycerin, where bioavailability discrepancies dictate dosing strategies tailored to individual patient needs.

Optimizing Therapeutic Strategies
Utilizing bioavailability data empowers clinicians to devise tailored pharmacotherapeutic regimens. Equations facilitating dose calculation based on bioavailability, dosage, and clearance offer a systematic approach to dosage determination. Whether prescribing loading doses for treatment initiation or maintenance doses for long-term therapy, understanding bioavailability is paramount in achieving desired clinical outcomes while mitigating adverse effects.
Empowering Precision Medicine
In the intricate realm of pharmacology, bioavailability emerges as a linchpin guiding therapeutic precision. Its multifaceted interplay with drug administration, clearance, and distribution underscores its significance in optimizing pharmacotherapeutic interventions. By unraveling the mysteries of bioavailability, clinicians can navigate the complex landscape of drug efficacy and safety, ushering in a new era of precision medicine tailored to individual patient needs.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Spatial Collapse: Pharmacologic Degradation of PDEδ to Disrupt Oncogenic KRAS Membrane Localization
PDEδ degradation disrupts KRAS membrane localization to collapse oncogenic signaling through spatial pharmacology rather than direct enzymatic inhibition.

Medicinal Chemistry & Pharmacology
Neumedics’ Integrated Innovation Model: Dr. Mark Nelson on Translating Drug Discovery into API Synthesis
Dr. Mark Nelson of Neumedics outlines how integrating medicinal chemistry with scalable API synthesis from the earliest design stages defines the next evolution of pharmaceutical development.

Medicinal Chemistry & Pharmacology
Exelixis Clinical Bioanalysis Leadership, Translational DMPK Craft, and the Kirkovsky Playbook
Senior Director Dr. Leo Kirkovsky brings a rare cross-modality perspective—spanning physical organic chemistry, clinical assay leadership, and ADC bioanalysis—to show how ADME mastery becomes the decision engine that turns complex drug systems into scalable oncology development programs.
Read More Articles
Zentalis Pharmaceuticals’ Clinical Strategy Architecture: Dr. Stalder on Data Foresight and Oncology Execution
Dr. Joseph Stalder of Zentalis Pharmaceuticals examines how predictive data integration and disciplined program governance are redefining the future of late-stage oncology development.
Policy Ignition: How Institutional Experiments Become Durable Global Evidence for Pharmaceutical Access
Global pharmaceutical access improves when IP, payment, and real-world evidence systems are engineered as interoperable feedback loops rather than isolated reforms.
Sepsis Shadow: Machine-Learning Risk Mapping for Stroke Patients with Bloodstream Infection
Regularized models like LASSO can identify an interpretable risk signature for stroke patients with bloodstream infection, enabling targeted, physiology-aligned clinical management.
Enduring Blockade: Five-Year Functional Antibody Persistence Against Emerging GII.4 and GII.17 Noroviruses
Natural infection with dominant GII noroviruses elicits long-lived functional antibodies, redefining immune durability in viral gastroenteritis.
Signal Switch: Stimuli-Responsive Nanoplatforms That Turn STING On Only Where Tumors Make Sense
Stimuli-responsive STING nanomedicine is an effort to make innate immune activation behave like a gated, tumor-local event rather than a body-wide inflammatory signal.