Intracellular protein delivery is at the frontier of molecular medicine, representing a transformative approach that enables the replacement of dysfunctional, poorly expressed, or absent proteins within living cells. This technology has profound implications across fields such as drug development, cell and stem cell engineering, genome editing, and precision oncology. By delivering functional proteins into cells, scientists can modulate key cellular processes that were previously inaccessible, opening avenues for treating diseases that were once deemed untreatable.
The promise of intracellular protein delivery lies in its ability to address “undruggable” targets, such as transcription factors or scaffolding proteins, which are challenging to approach with conventional small molecules. Unlike small molecules, proteins can engage complex intracellular pathways and directly regulate cellular behavior. However, despite its potential, intracellular protein delivery is a formidable challenge due to the structural and physicochemical properties of proteins. They are large, susceptible to rapid degradation, and typically lack the ability to permeate cellular membranes. Consequently, the development of efficient, minimally invasive, and biocompatible delivery methods is a high priority for advancing the field.
Intracellular Delivery: Existing Strategies and Their Limitations
Several physical and chemical strategies have been developed to transport proteins into cells, including electroporation, microinjection, and cell-penetrating peptides (CPPs). Physical methods, such as electroporation and microinjection, involve the direct injection of proteins into cells or the temporary disruption of cell membranes. While these techniques can achieve high delivery efficiency, their limitations are significant. They are disruptive, often damaging cell membranes and cellular components, which restricts their application to research settings with limited scalability for therapeutic applications.
Chemical methods, particularly CPPs, offer a less invasive approach by exploiting peptide sequences that can facilitate protein entry into cells. These peptides typically deliver proteins through endocytosis, a process whereby proteins are engulfed by the cell membrane and contained within endosomes. However, endosomal entrapment remains a major barrier to effective protein delivery, as proteins often fail to escape the endosome and become degraded by cellular enzymes before they reach their target site in the cytoplasm or nucleus. To overcome endosomal trapping, additional design elements, such as endosomal membrane disruptors, are often required, but these can introduce toxicity and complexity to the delivery system.
In response to these limitations, researchers have turned to nanoscale carriers, including liposomes and virus-like particles, which encapsulate proteins and protect them during delivery. While these nanocarriers can be tailored to enhance delivery efficiency, they still frequently encounter endosomal barriers, leading to reduced protein activity and potential toxicity. Consequently, the need for innovative, non-endocytic strategies has catalyzed interest in molecular carriers that can deliver proteins directly into the cytosol without requiring endosomal escape.
The Emergence of Molecular Carriers: Leveraging Superchaotropic Ions for Protein Transport
In the search for effective intracellular delivery methods, molecular carriers based on superchaotropic ions have emerged as a promising alternative. Superchaotropic ions possess unique physicochemical properties that enable them to permeate lipid bilayers directly, bypassing the endocytic pathway. This approach is particularly advantageous for proteins, as it avoids the complications of endosomal entrapment and degradation, allowing for efficient cytosolic delivery.
Among the most promising superchaotropic ions are inorganic boron cluster (IBC) anions, which offer several distinctive advantages. Unlike traditional amphiphilic carriers, which rely on a balance between hydrophilic and lipophilic segments, IBCs are inherently water-soluble and possess a high affinity for hydrophobic interfaces, including lipid membranes. This dual affinity arises from their unique ionic properties, enabling them to form stable interactions with both proteins and lipid bilayers. Boron clusters, such as dodecaborate (B12X12) or decaborate (B10X10) anions, have been extensively studied for their chemical stability, low toxicity, and capacity to interact with biological membranes. These properties make IBCs highly suitable for delivering functional proteins directly into the cytoplasm.
Recent research has highlighted the effectiveness of a specific brominated dodecaborate, B12Br11OCH2CH2CH3 (referred to as IBC-Pr), in facilitating the delivery of cytochrome c (CYC) into cells. CYC, a positively charged protein with a critical role in apoptosis initiation, serves as an ideal candidate for evaluating the capabilities of IBC-Pr as a molecular carrier. The unique properties of IBC-Pr, including its chaotropic nature and high affinity for cellular membranes, enable it to bind to CYC and facilitate its passage through lipid bilayers without relying on endocytosis. This innovative approach represents a significant departure from conventional delivery systems, with the potential to overcome longstanding challenges in intracellular protein transport.
Mechanistic Insights: The Chaotropic Effect and Protein-Carrier Interactions
The efficacy of IBC-Pr as a molecular carrier for CYC is rooted in the chaotropic effect, a phenomenon whereby certain ions disrupt the ordered structure of water, enhancing their interactions with organic interfaces such as proteins and lipid bilayers. Chaotropic ions like boron clusters exhibit high affinities for hydrophobic and positively charged regions on protein surfaces, allowing them to form stable, non-covalent complexes with proteins. For IBC-Pr, these interactions are both enthalpically and entropically favorable, as evidenced by isothermal titration calorimetry experiments, which indicate that binding is driven by both electrostatic and hydrophobic forces.
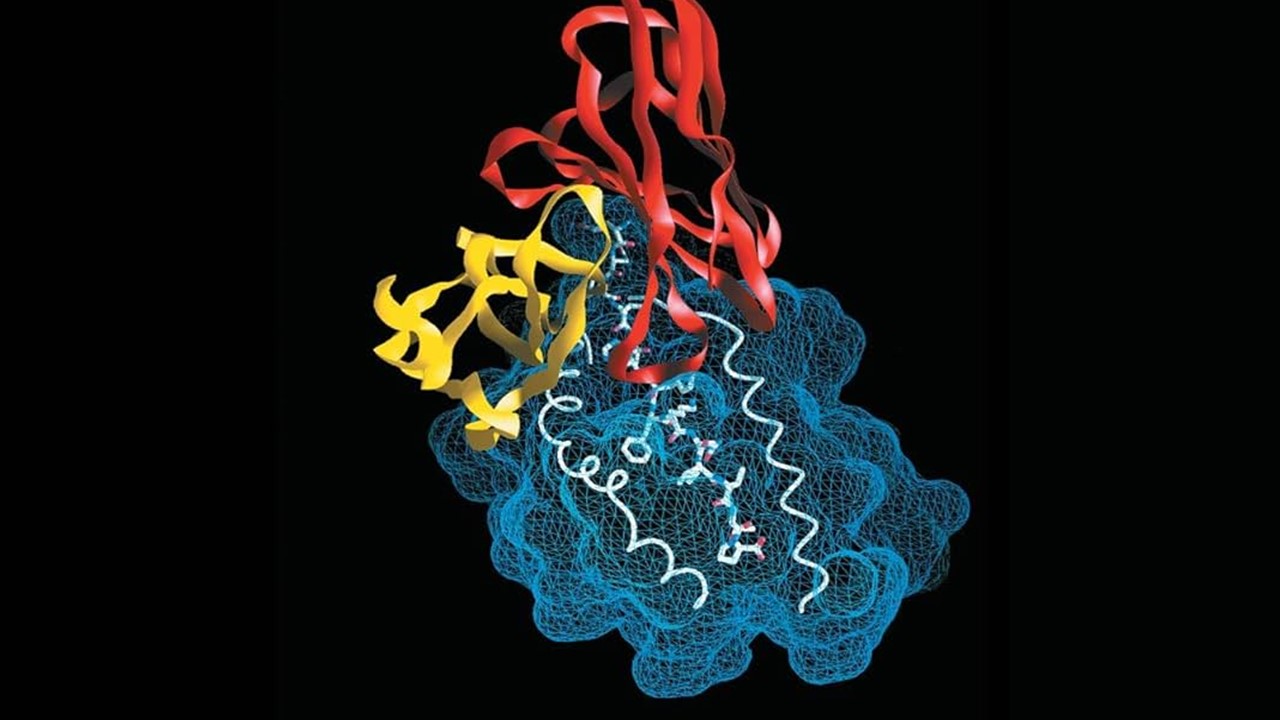
Studies of IBC-Pr binding to CYC have revealed two primary binding sites on the protein surface, located within concave hydrophobic regions that contain positively charged amino acid residues. These regions, identified through molecular simulations and fluorescence spectroscopy, offer ideal binding sites for IBC-Pr due to their favorable electrostatic and hydrophobic properties. The resulting protein-carrier complex, stabilized through chaotropic interactions, enables CYC to cross the lipid bilayer and enter the cytoplasm.
Fluorescence experiments have shown that binding with IBC-Pr enhances the fluorescence intensity of tryptophan residues within CYC, suggesting a shift in their microenvironment as they interact with the polarizable boron cluster. This shift is consistent with the chaotropic effect, which promotes stable, reversible binding without compromising the structural integrity of the protein. Importantly, the binding of IBC-Pr to CYC is sufficiently dynamic to allow for protein release within the cytoplasm, where CYC can initiate its apoptotic signaling functions.
Preserving Protein Structure and Function: A Key Advantage of IBC-Pr
One of the primary concerns in protein delivery is maintaining the structural and functional integrity of the protein after crossing the cellular membrane. For therapeutic applications, any alteration in protein conformation or activity could diminish the efficacy of the treatment. In the case of CYC, maintaining its structural integrity is essential, as the protein’s role in apoptosis relies on its ability to engage specific cellular pathways.
Circular dichroism (CD) spectroscopy and enzyme assays have demonstrated that IBC-Pr binding does not significantly alter the secondary structure or catalytic activity of CYC. These findings indicate that the boron cluster carrier can deliver CYC into cells without inducing denaturation or aggregation, which are common challenges in protein delivery. Additionally, zeta potential measurements have shown that IBC-Pr binding reduces the positive surface charge of CYC, partially neutralizing it and further stabilizing the protein-carrier complex. The preservation of CYC’s functional properties following IBC-Pr-mediated delivery highlights the carrier’s ability to facilitate efficient transmembrane transport without compromising protein activity.
The bioactivity of CYC after delivery is particularly relevant for its role in apoptosis. As an apoptosis-inducing protein, CYC must retain its capacity to initiate the caspase activation cascade in the cytoplasm. Enzyme assays have confirmed that CYC delivered by IBC-Pr retains its peroxidase activity, which is crucial for its apoptotic function. This retention of bioactivity underscores the potential of IBC-Pr as a molecular carrier for therapeutic proteins, as it can facilitate effective delivery while preserving the protein’s therapeutic function.
Experimental Validation: Transmembrane Transport and Cellular Uptake
The ability of IBC-Pr to facilitate transmembrane transport was evaluated in both artificial and biological membrane models. In experiments with phospholipid bilayer vesicles, IBC-Pr successfully delivered CYC across lipid bilayers, as indicated by the release of a fluorescent dye from the vesicles upon CYC uptake. These in vitro experiments provided a controlled environment to study the transport efficiency of IBC-Pr, demonstrating its capacity to facilitate protein delivery without disrupting membrane integrity.
To confirm the effectiveness of IBC-Pr in cellular models, studies were conducted in HeLa cells, where CYC was labeled with a fluorescent tag to track its intracellular localization. Confocal microscopy revealed that CYC delivered by IBC-Pr permeated the cellular membrane and distributed evenly throughout the cytoplasm and nucleus, indicating successful intracellular delivery. Flow cytometry analysis further quantified the efficiency of cellular uptake, showing a significant increase in fluorescence intensity when CYC was delivered with IBC-Pr, compared to CYC alone. Notably, the uptake efficiency was unaffected by inhibitors of energy-dependent pathways, confirming that IBC-Pr mediates energy-independent, direct permeation of the protein across the membrane.
Therapeutic Implications: Inducing Apoptosis in Target Cells
The therapeutic potential of IBC-Pr-mediated protein delivery is exemplified by its ability to induce apoptosis in cancer cells. As a key mediator of apoptosis, CYC initiates the apoptotic signaling cascade by binding to apoptotic protease activating factor 1 (Apaf-1) in the cytoplasm. In HeLa cell experiments, CYC delivered by IBC-Pr triggered dose-dependent apoptosis, with an IC50 value of 7.5 μM for inducing cell death after 48 hours. This cytotoxic effect was not observed with free CYC, highlighting the efficacy of IBC-Pr in facilitating intracellular delivery and preserving bioactivity.
Further analysis using the Annexin V-FITC/PI apoptosis assay confirmed the apoptotic effect of CYC delivered by IBC-Pr. HeLa cells treated with the CYC/IBC-Pr complex exhibited characteristic markers of apoptosis, including membrane blebbing and DNA fragmentation. This apoptotic response was comparable to or greater than that observed with traditional microheterogeneous carriers, such as nanoparticles, which require additional steps for loading and release. The ability of IBC-Pr to directly permeate the lipid bilayer and deliver functional CYC represents a streamlined approach with potential applications in cancer therapy and other fields requiring targeted cell death.
Advancing Molecular Delivery: The Future of Boron Cluster Carriers in Medicine
The development of IBC-Pr as a molecular carrier for intracellular protein delivery has far-reaching implications for molecular medicine. Its unique properties, including high water solubility, low toxicity, and reversible binding, make it a versatile platform for delivering therapeutic proteins directly to the cytoplasm. Unlike traditional nanocarriers, IBC-Pr does not require complex formulation or endosomal escape strategies, simplifying the delivery process and enhancing bioavailability.
Looking forward, the adaptability of boron cluster carriers offers exciting opportunities for future applications. Modifying the chemical structure of boron clusters could enable targeted delivery to specific cell types or subcellular compartments, enhancing selectivity and therapeutic efficacy. Additionally, the potential to deliver a broader range of proteins, particularly positively charged ones, could expand the therapeutic scope of this technology to include a wider array of intracellular targets.
As research progresses, boron cluster-based molecular carriers like IBC-Pr may pave the way for a new generation of intracellular delivery systems that overcome the limitations of traditional approaches. By enabling efficient, direct delivery of functional proteins, these carriers have the potential to transform the treatment of diseases that rely on precise modulation of intracellular processes. The promising results of IBC-Pr in delivering cytochrome c and initiating apoptosis in cancer cells suggest that boron cluster carriers could become valuable tools in the quest to unlock new frontiers in molecular medicine.
Study DOI: https://doi.org/10.1073/pnas.2407515121
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES
Register your interest [here] at Proventa International’s Clinical Operations and Clinical Trials Supply Chain Strategy Meeting this 14th of November 2024 at Le Meridien Boston Cambridge, Massachusetts, USA to engage with thought leaders and like-minded peers on the latest developments in the clinical space and regulatory affairs.

Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.
Medicinal Chemistry & Pharmacology
Coprocessed for Compression: Reengineering Metformin Hydrochloride with Hydroxypropyl Cellulose via Coprecipitation for Direct Compression Enhancement
In manufacturing, minimizing granulation lines, drying tunnels, and multiple milling stages reduces equipment costs, process footprint, and energy consumption.

Medicinal Chemistry & Pharmacology
Decoding Molecular Libraries: Error-Resilient Sequencing Analysis and Multidimensional Pattern Recognition
tagFinder exemplifies the convergence of computational innovation and chemical biology, offering a robust framework to navigate the complexities of DNA-encoded science