Medicinal Chemistry & Pharmacology
Designing Better Sugar Stoppers: Engineering Selective α-Glucosidase Inhibitors via Fragment-Based Dynamic Chemistry
One of the most pressing challenges in anti-diabetic therapy is reducing the unpleasant and often debilitating gastrointestinal side effects that accompany α-amylase inhibition.
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.
Coprocessed for Compression: Reengineering Metformin Hydrochloride with Hydroxypropyl Cellulose via Coprecipitation for Direct Compression Enhancement
In manufacturing, minimizing granulation lines, drying tunnels, and multiple milling stages reduces equipment costs, process footprint, and energy consumption.
Decoding Molecular Libraries: Error-Resilient Sequencing Analysis and Multidimensional Pattern Recognition
tagFinder exemplifies the convergence of computational innovation and chemical biology, offering a robust framework to navigate the complexities of DNA-encoded science
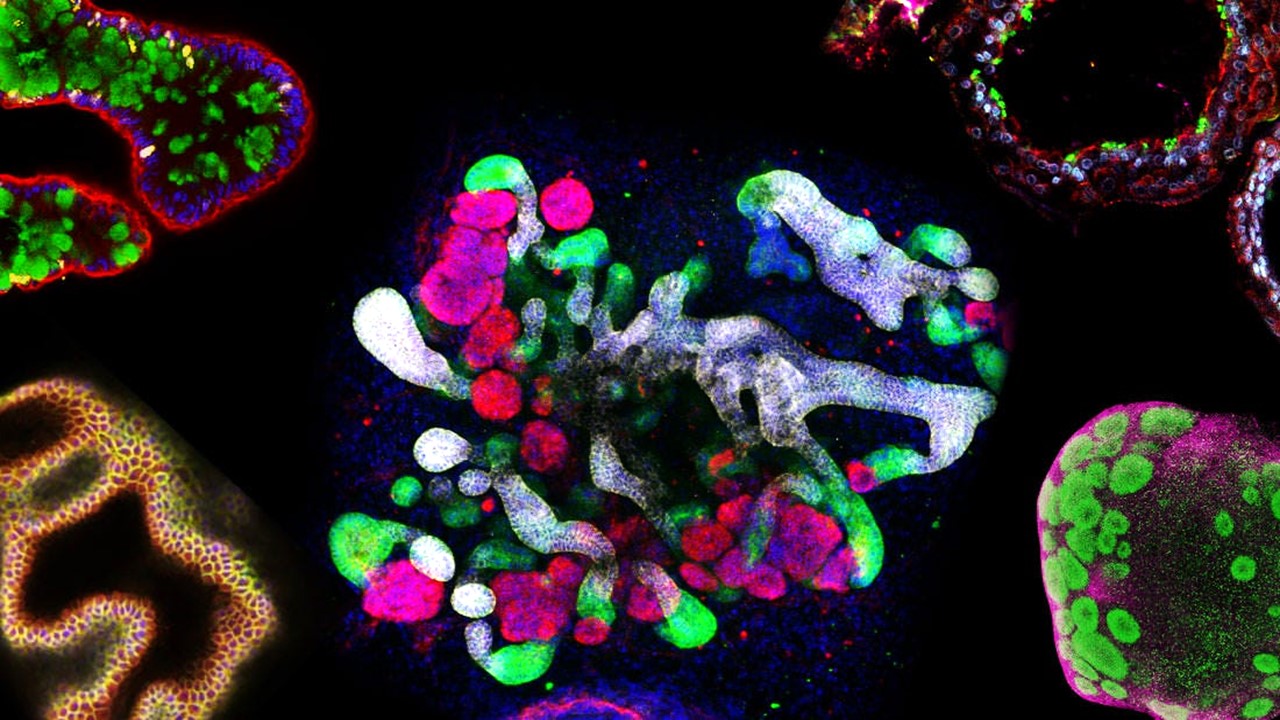
Microphysiological Systems: Pioneering the Next Frontier in Precision Pharmacology and Drug Development
Microphysiological systems provide human-relevant data to address pharmacology challenges and streamline approvals for complex cases like rare diseases.
Breaking Barriers in Molecular Dynamics: The Asynchronous Replica Exchange Revolution
Molecular dynamics simulations have been instrumental in exploring molecular interactions, providing atomic-level understanding of phenomena such as protein folding, ligand binding, and enzymatic catalysis.
Sneak Peak to Drug Behavior: The Fractured Foundations of Allometric Scaling in Modern Pharmacology
As the Darwinian view ascends, the future of pharmacokinetics lies not in defending old paradigms, but in forging new ones grounded in the irreducible diversity of life.
Advanced ADME Optimization: Unlocking the Therapeutic Potential of Peptide Xenobiotics
Peptides stand at the frontier of drug development, poised to exploit their unique blend of specificity and adaptability.
Matrix Lipidomics: Decoding Photosynthetic Supercomplexes Through Neutron Scattering and Molecular Profiling
The synthesis of neutron scattering, lipidomics, and photochemical analyses redefines PSI as a lipid-protein supercomplex, where every molecular component is a deliberate player in the photosynthetic symphony.
Molecular Alchemy: How MOSES is Redefining Drug Discovery in the Age of AI
MOSES serves as both compass and crucible, guiding researchers through chemical space while rigorously testing their innovations.