In the relentless pursuit of novel therapeutic agents, the field of drug discovery faces a pressing challenge: the scarcity of new chemical entities (NCEs) that exhibit both efficacy and safety profiles. In response, researchers have turned to computer-assisted de novo molecular design, a burgeoning field that harnesses the power of computational algorithms to generate innovative molecules with desirable activity profiles. Through this approach, researchers aim to overcome the limitations of traditional drug discovery methods and usher in a new era of pharmacological innovation.
The Landscape of De Novo Design
Traditionally, drug design strategies have relied on either receptor-based or ligand-based approaches. While receptor-based design focuses on the molecular interactions within a target binding site, ligand-based methods exploit chemical similarity principles to generate novel compounds. The latter approach, in particular, has gained traction for its ability to facilitate scaffold hopping and template mimetics generation without the need for explicit receptor models.
Receptor-based design in drug discovery revolves around understanding and manipulating the molecular interactions occurring within the binding site of a target macromolecule, typically a protein. This approach entails studying the three-dimensional structure of the target and designing molecules that specifically interact with it to elicit a desired biological response. In contrast, ligand-based methods take a different approach, leveraging the concept of chemical similarity to generate novel compounds without relying on detailed knowledge of the target’s structure. Instead, these methods analyze the chemical and pharmacophore features of known ligands and use them as templates to design new molecules with similar properties. By exploiting similarities in chemical structure or pharmacological activity, ligand-based methods facilitate the exploration of chemical space and the discovery of potentially therapeutic compounds, even in cases where the target’s structure is unknown or poorly characterized.
Challenges in Target Prediction
Predicting the polypharmacological activities of novel compounds poses a significant challenge in drug discovery. While promiscuity among drug targets can lead to both therapeutic benefits and adverse effects, existing target prediction tools often struggle to accurately identify the diverse pharmacological profiles of de novo-designed molecules. This limitation underscores the need for innovative approaches to target prediction that can accommodate the structural novelty of NCEs.
Introducing SPiDER
In response to the limitations of existing target prediction methods, researchers have developed SPiDER, a novel algorithm that leverages fuzzy molecular representations to infer potential drug targets. By combining multiple molecular models and employing statistical evaluations, SPiDER offers a comprehensive and accurate approach to target prediction for de novo-designed compounds.
SPiDER, or the SOM-based Prediction of Drug Equivalence Relationships, represents a sophisticated advancement in bioinformatics and computer science tailored specifically for drug discovery. At its core, SPiDER utilizes self-organizing maps (SOMs), which are a type of artificial neural network, to cluster and analyze large datasets of pharmaceutically relevant compounds. These SOMs enable the creation of a high-dimensional map where similar compounds are grouped together in close proximity. SPiDER leverages this capability to predict potential drug targets for newly designed compounds by comparing them to reference compounds with known target profiles. Notably, SPiDER employs chemically abstract molecular representations, such as pharmacophoric feature descriptors, to capture subtle functional relationships between compounds, thereby allowing for scaffold hopping and the identification of targets unrelated to the original template. Furthermore, SPiDER incorporates statistical methods to estimate the significance of its predictions, mitigating false-positive results and providing researchers with confident assessments of target binding.
Validation and Prospective Applications
Predicting both on-target and off-target interactions is crucial in drug discovery to optimize efficacy and minimize adverse effects. By accurately identifying potential interactions with both intended targets and unintended off-targets, researchers can design safer and more effective therapeutics.
Validation studies have demonstrated the efficacy of SPiDER in predicting both on-target and off-target interactions for a variety of pharmacologically active compounds. Notably, SPiDER’s ability to identify targets for structurally dissimilar molecules highlights its potential for guiding the design of novel therapeutics. Moreover, prospective applications of SPiDER in de novo drug design hold promise for accelerating the discovery of new chemical entities with tailored pharmacological profiles.
Onwards Next-Gen Therapeutics
As the landscape of drug discovery continues to evolve, computational tools like SPiDER are poised to play a pivotal role in reshaping the field. By bridging the gap between computational modeling and experimental validation, these innovative approaches offer new avenues for accelerating the discovery of safe and effective therapeutics. As researchers continue to push the boundaries of computer-assisted drug design, the future of pharmacological innovation appears brighter than ever before.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts

Drug Discovery Biology
Proteolytic Rewriting: Engineering Controlled Absence of Pathogenic Protein Persistence
Targeted protein degradation transforms drug therapy by engineering the cellular machinery to erase, rather than merely inhibit, pathogenic proteins.

Drug Discovery Biology
Open Nucleotides: Grant-Driven Infrastructure for Equitable mRNA Vaccine Manufacturing
By developing accessible cap analogs and RNA raw materials, Hongene Biotech, guided by David Butler’s expertise in nucleotide chemistry and supported by the Gates Foundation, is reshaping the molecular infrastructure that underpins global mRNA vaccine equity.
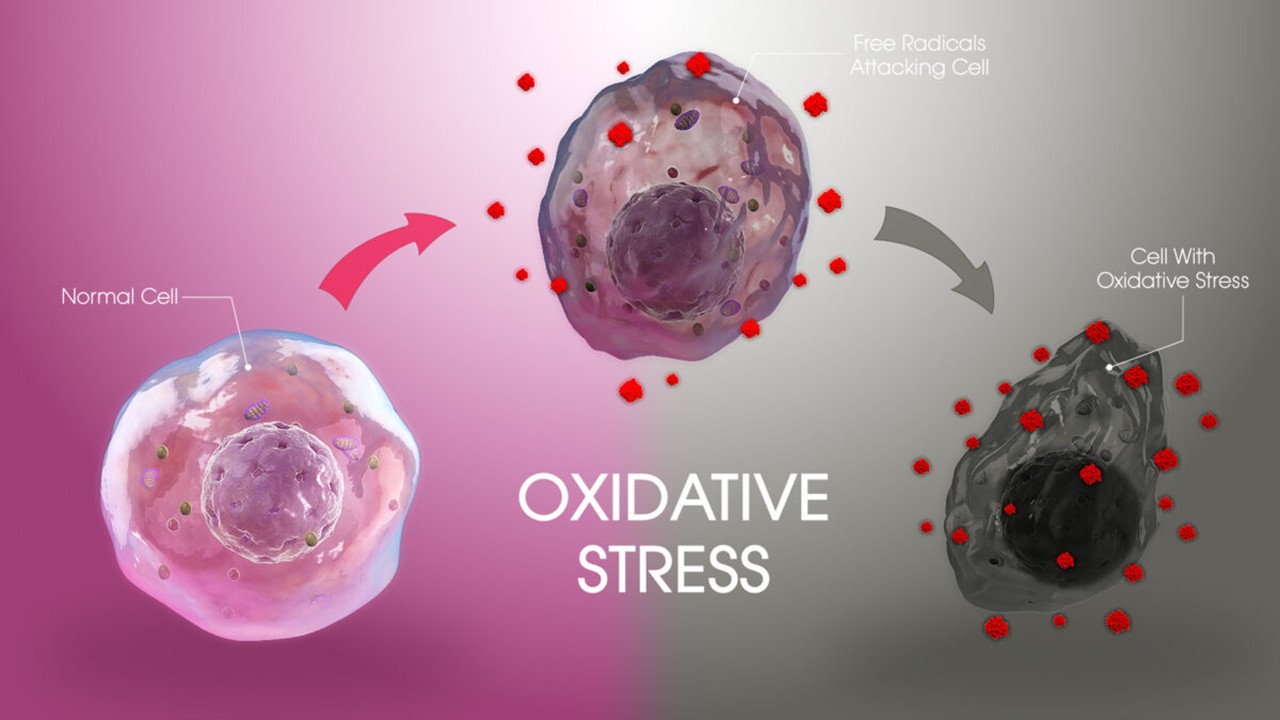
Drug Discovery Biology
Redox Messengers: How Endogenous Gasotransmitters Rewire Vascular Biology to Resist Age-Driven Oxidative Stress
Gasotransmitters provide a biologically sophisticated means of counteracting age-related oxidative stress and preserving vascular resilience.
Drug Discovery Biology
Aptamer Signal Dynamics: Engineering Nucleic Acid Recognition Systems for High-Fidelity, Multi-Modal Biosensing
Aptamers redefine biosensing by pairing programmable molecular recognition with versatile transduction strategies capable of detecting clinically relevant biomarkers with exceptional fidelity.
Read More Articles
Spatial Collapse: Pharmacologic Degradation of PDEδ to Disrupt Oncogenic KRAS Membrane Localization
PDEδ degradation disrupts KRAS membrane localization to collapse oncogenic signaling through spatial pharmacology rather than direct enzymatic inhibition.
Neumedics’ Integrated Innovation Model: Dr. Mark Nelson on Translating Drug Discovery into API Synthesis
Dr. Mark Nelson of Neumedics outlines how integrating medicinal chemistry with scalable API synthesis from the earliest design stages defines the next evolution of pharmaceutical development.
Zentalis Pharmaceuticals’ Clinical Strategy Architecture: Dr. Stalder on Data Foresight and Oncology Execution
Dr. Joseph Stalder of Zentalis Pharmaceuticals examines how predictive data integration and disciplined program governance are redefining the future of late-stage oncology development.
Exelixis Clinical Bioanalysis Leadership, Translational DMPK Craft, and the Kirkovsky Playbook
Senior Director Dr. Leo Kirkovsky brings a rare cross-modality perspective—spanning physical organic chemistry, clinical assay leadership, and ADC bioanalysis—to show how ADME mastery becomes the decision engine that turns complex drug systems into scalable oncology development programs.
Policy Ignition: How Institutional Experiments Become Durable Global Evidence for Pharmaceutical Access
Global pharmaceutical access improves when IP, payment, and real-world evidence systems are engineered as interoperable feedback loops rather than isolated reforms.
Sepsis Shadow: Machine-Learning Risk Mapping for Stroke Patients with Bloodstream Infection
Regularized models like LASSO can identify an interpretable risk signature for stroke patients with bloodstream infection, enabling targeted, physiology-aligned clinical management.







