Dr. Stanley “Stan” Satz revolutionized the field of nuclear medicine in the 1970s by introducing a reliable method for isolating the potent alpha-emitting radioisotope actinium-225. Serving as the inaugural Chairman of Advanced Innovative Partners (AIP), Dr. Satz played a pivotal role in positioning actinium-225 as a promising radionuclide for targeted alpha therapy.
Alpha Particles: In A Nutshell
Alpha particles constitute composite particles formed by tightly binding two protons and two neutrons together. They are emitted during a specific form of radioactive decay known as alpha decay, originating from the nucleus of certain radionuclides. Essentially, an alpha particle is identical to the nucleus of a normal helium atom with an atomic mass of four, effectively representing a doubly ionized helium atom.

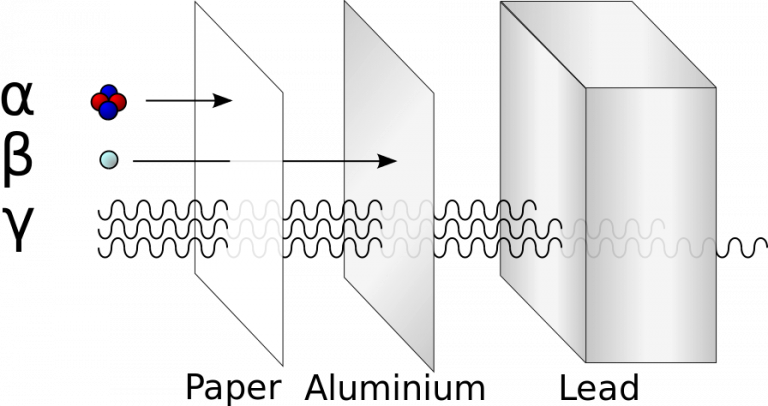
Termed alpha radiation or alpha rays, these particles were the earliest form of nuclear radiation to be discovered, with the subsequent identification of beta particles and gamma rays. Notably, alpha particles possess a double positive charge, substantial mass compared to beta particles, and relative slowness, rendering them highly ionizing. This characteristic, combined with their ability to cause multiple ionizations within a very confined distance, signifies their potential for inflicting greater biological damage for a given amount of deposited energy.
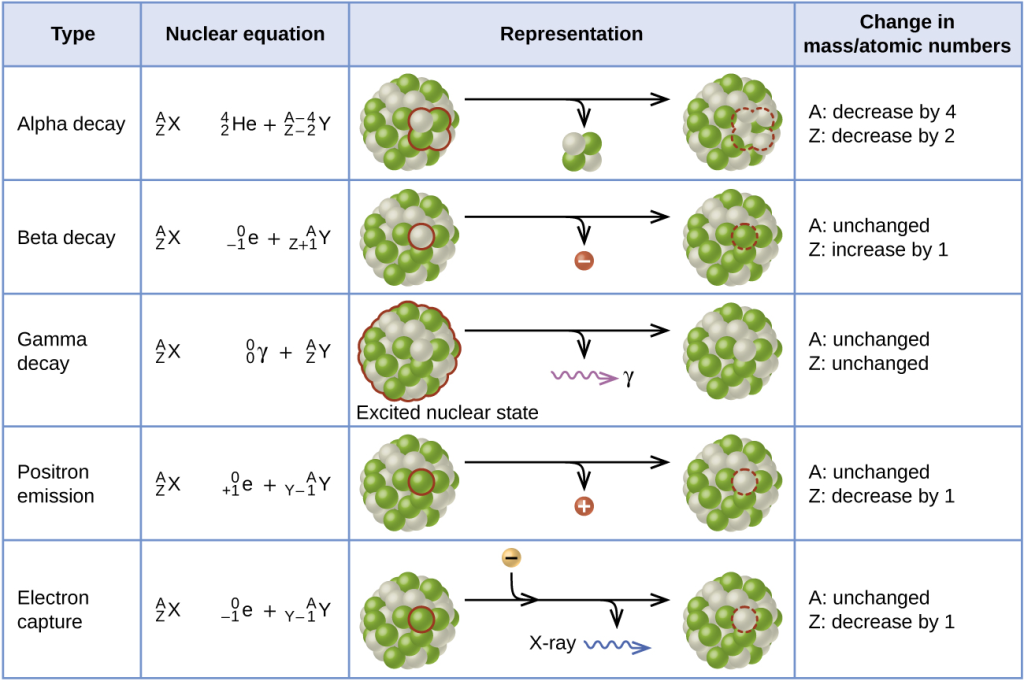
While alpha particles cannot penetrate the outer layer of dead cells on the skin, they can inflict damage to the cornea of the eye. The safety concern with alpha-particle radiation arises primarily when the radioactive decay originates from an atom already within the body or a cell. Inhaling, ingesting, or entry through a wound are scenarios where alpha-particle emitters become particularly hazardous.
Numerous alpha emitters are naturally present in the environment, emanating from radionuclides like uranium-238, radium-226, and other members of the naturally occurring uranium, thorium, and actinium decay series. These elements exist in varying concentrations in rocks, soils, and water.
Artificial sources of alpha particles are synthetically produced and include the radioisotopes of elements such as plutonium, americium, curium, and californium. Typically generated in a nuclear reactor through neutron absorption by various uranium radioisotopes, these artificial sources contribute to the understanding and application of alpha particle characteristics in various scientific and technological contexts.
Alpha Particles in Oncotherapeutics
The potential of alpha particles in cancer treatment lies in their ability to deposit significant energy within a short range. This precise cell-killing capability offers the advantage of sparing surrounding healthy tissues. Historically, the utilization of alpha emitters for therapy has been hampered by inadequate production technology. Dr. Satz’s breakthrough not only transformed the landscape but also addressed the historical limitations associated with harnessing alpha emitters for therapeutic purposes.

Through pioneering research, Dr. Satz achieved the optimization of actinium-225 isolation from thorium-229 and radium-225 source materials. His breakthroughs in actinium purification resulted in the attainment of a high specific activity crucial for clinical applications. Furthermore, Satz conducted a comprehensive characterization of actinium’s decay properties and its daughter radionuclides. “Actinium-225’s 10-day half-life and potent 4 alpha particles per decay make it ideal for molecularly targeted therapy,” said Dr. Satz. “But realizing its full potential required developing scalable production and isolation methods.”
In collaboration with the U.S. Department of Energy, Dr. Satz successfully generated the first GMP-grade actinium-225 designed for human use. He holds patents related to actinium-225 production, establishing a commercial avenue that allows this alpha emitter to be accessible to research institutions and pharmaceutical companies.
Actinium-225: Tumor-Targeting and Beyond
Presently, actinium-225 is demonstrating significant potential in clinical trials for the treatment of prostate cancer, leukemia, and other cancers. This is particularly evident when it is coupled with tumor-targeting molecules such as antibodies and peptides. Ongoing research conducted by AIP and others suggests that actinium-225 has the capability to enhance and prolong the lives of cancer patients worldwide. Reflecting on this progress, Dr. Satz expressed, “Seeing actinium-225 emerge as a versatile platform for precision alpha therapy has been extremely gratifying. None of this would have been possible without taking the initial step to establish scalable production.”
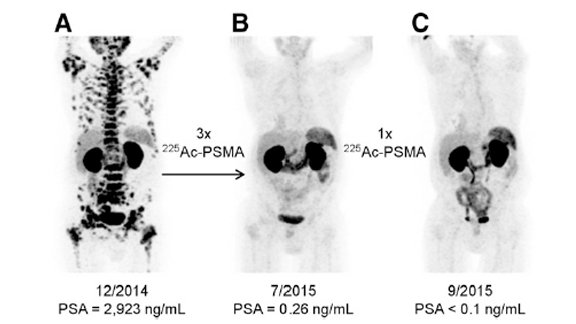
Dr. Satz’s foresight and tenacity have not only unlocked the potential of actinium-225 but have also provided a beacon of hope for cancer patients globally. His groundbreaking efforts have paved the way for a new era of targeted alpha therapies, reshaping the landscape of nuclear medicine.
About Advanced Innovative Partners (AIP)
Advanced Innovative Partners (AIP) emerges as a prominent global clinical-stage biotechnology company, standing at the forefront of diagnostic and therapeutic advancements in the realm of targeted radiation. Founded in 2017, AIP boasts a skilled team with expertise spanning biotechnology, nuclear medicine, and molecular biology. Their commitment lies in the development of next-generation diagnostic and therapeutic radiopharmaceuticals, addressing critical needs in oncology, rare pediatric diseases, infectious diseases, and biomedical countermeasures.

At the heart of AIP’s operations is a robust pipeline, extending its reach to breast, lung, brain, and solid tumor cancers, as well as rare diseases. AIP navigates this expansive landscape with a reliable, secure global supply chain and a collaborative network, ensuring the seamless progression of their innovative products from development to delivery.
With lead programs advancing through Phase I/II/III clinical trials, AIP positions itself as a leader in the emerging market space of radiopharmaceutical diagnostics and therapeutics. The company’s success is underpinned by patented platform technologies that underscore its commitment to excellence in treatment and clinical management. AIP’s approach is product-centric, reflected in the creation of a pipeline comprising multiple drugs strategically targeting areas with significant market opportunities.
AIP’s mission revolves around expediting the discovery, development, and global availability of transformative therapies. Their focus extends beyond innovation, emphasizing the importance of rigorous clinical trials mandated by regulatory bodies. By navigating these regulatory pathways and obtaining approvals, AIP aims to make its investigational products accessible to patients worldwide.
Central to AIP’s identity is a dedication to pioneering targeted therapies and advancing disease detection and treatment. This commitment unfolds through a comprehensive, integrated approach encompassing proprietary tech platforms, strategic partnerships, and an unwavering patient-centric focus. AIP’s ultimate goal is to profoundly impact patient journeys by enabling accurate diagnoses at an earlier stage and providing treatments that are not only more powerful but also gentler, thereby offering patients the best chance for improved outcomes. In essence, AIP aspires to make a meaningful difference for individuals grappling with complex, aggressive, rare, and infectious diseases worldwide.

Learn more about AIP from their Official Website.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Invisible Couriers: How Lab-on-Chip Technologies Are Rewriting the Future of Disease Diagnosis
The shift from benchtop Western blots to on-chip, real-time protein detection represents more than just technical progress—it is a shift in epistemology.

Medicinal Chemistry & Pharmacology
Designing Better Sugar Stoppers: Engineering Selective α-Glucosidase Inhibitors via Fragment-Based Dynamic Chemistry
One of the most pressing challenges in anti-diabetic therapy is reducing the unpleasant and often debilitating gastrointestinal side effects that accompany α-amylase inhibition.

Medicinal Chemistry & Pharmacology
Into the Genomic Unknown: The Hunt for Drug Targets in the Human Proteome’s Blind Spots
The proteomic darkness is not empty. It is rich with uncharacterized function, latent therapeutic potential, and untapped biological narratives.

Medicinal Chemistry & Pharmacology
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.











