As we mark the passage of almost 53 years since the inception of the “war on cancer,” reflections on the progress and challenges in cancer drug development illuminate both triumphs and hurdles. This pivotal endeavor, catalyzed by the National Cancer Act of 1971 (December 23), has seen remarkable strides propelled by advancements in supportive care, drug innovations, and early detection methods.
However, amidst these achievements loom the shadows of a modern era marked by shifting environmental dynamics, lifestyle modifications, and dietary changes, potentially fueling a concerning surge in early-onset cancers, thus heralding what some experts perceive as an emerging global health crisis.
The Evolution of Anticancer Armamentarium: A Historical Perspective
Conventional Chemotherapy
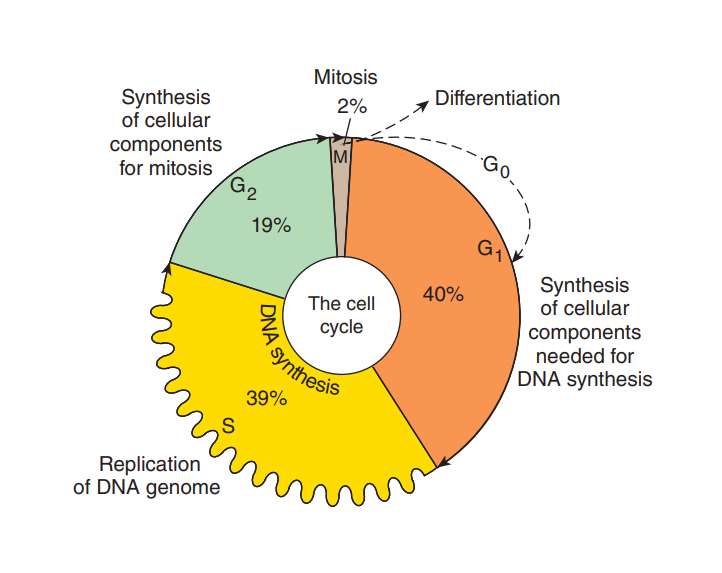
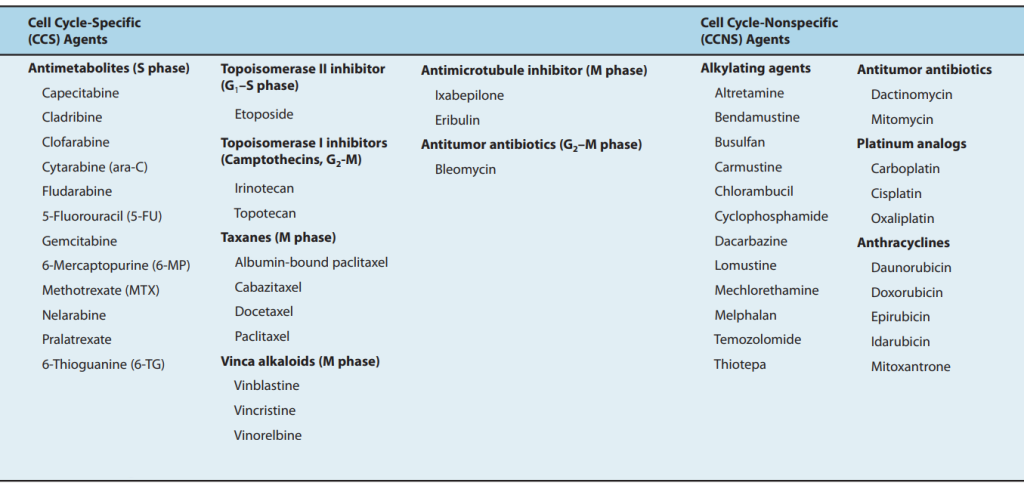
Since its inception in the late 1940s, conventional chemotherapy has stood as a cornerstone in cancer treatment, wielding a diverse arsenal comprising alkylating agents, antimetabolites, natural products, and hormonal interventions. Despite its efficacy in targeting rapidly dividing cancer cells, its non-specific nature indiscriminately affects healthy tissues, leading to debilitating side effects. Despite these limitations, cytotoxic chemotherapy remains a prevalent modality in contemporary oncology.
Targeted Therapies
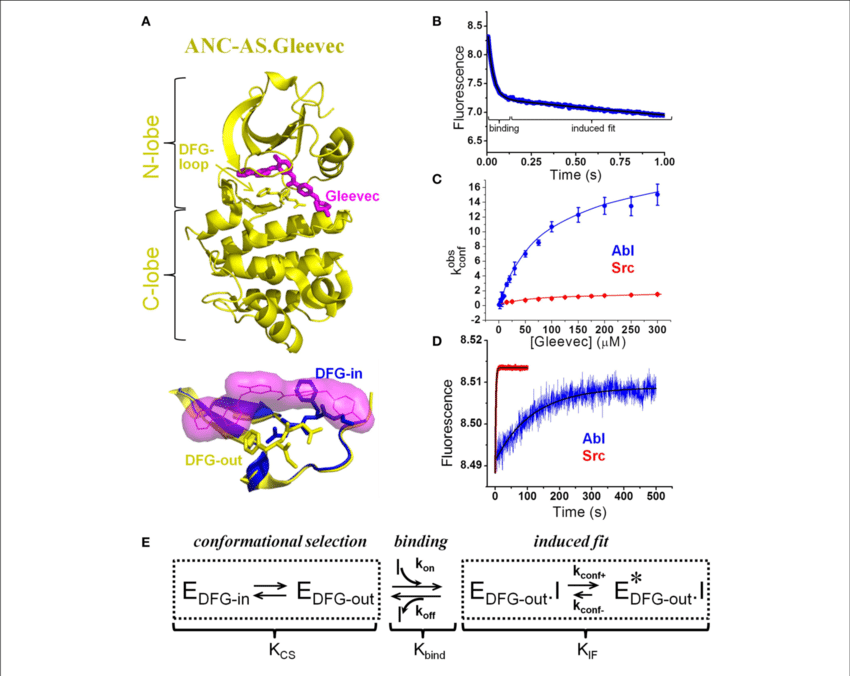
The advent of targeted therapies in the late 1980s heralded a paradigm shift in cancer treatment, premised on the identification and exploitation of unique molecular targets within cancer cells. Exemplifying this approach is the success story of imatinib mesylate (Gleevec), a BCR-ABL tyrosine kinase inhibitor, which revolutionized the treatment landscape for certain malignancies. However, while targeted therapies have showcased promising results in preclinical models, their translation into clinical efficacy has been inconsistent, underscoring the complexity of cancer biology.
Immunotherapy and Gene Editing
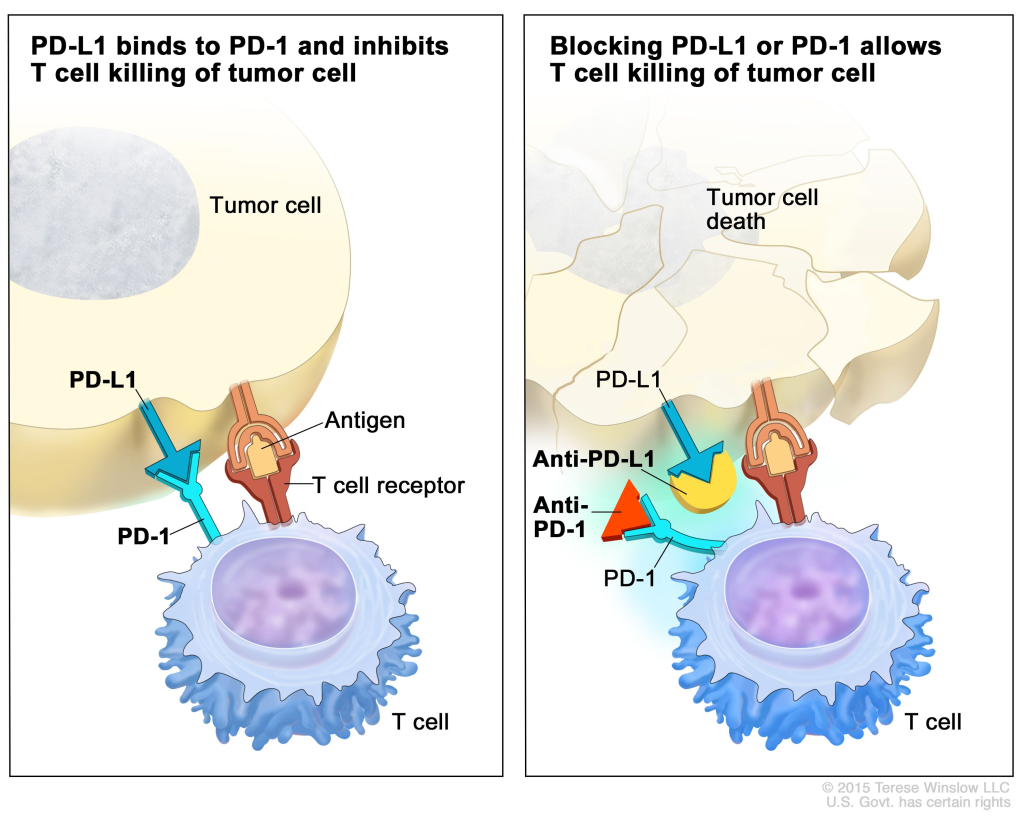
Immunotherapy, epitomized by immune checkpoint inhibitors and CAR-T cell therapy, represents a revolutionary frontier in cancer therapeutics, harnessing the body’s immune system to combat malignancies. While yielding unprecedented responses in select patient cohorts, challenges such as long-term toxicity and limited efficacy in solid tumors temper the enthusiasm surrounding these modalities.
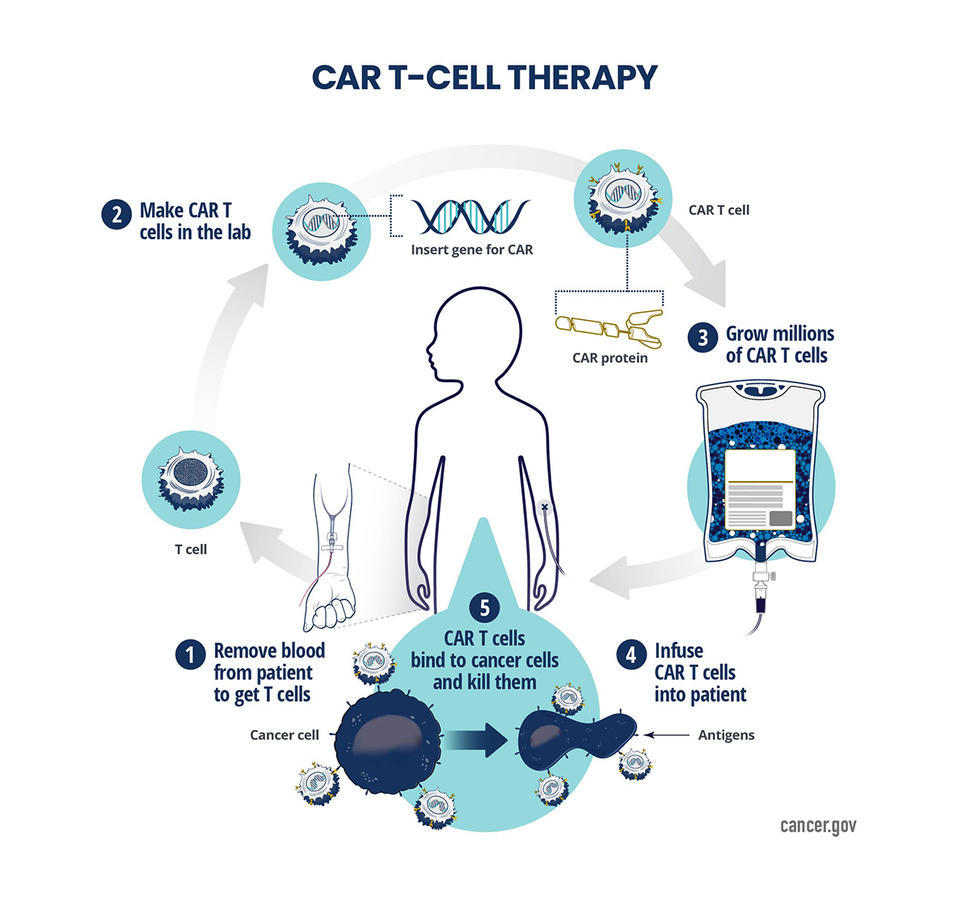
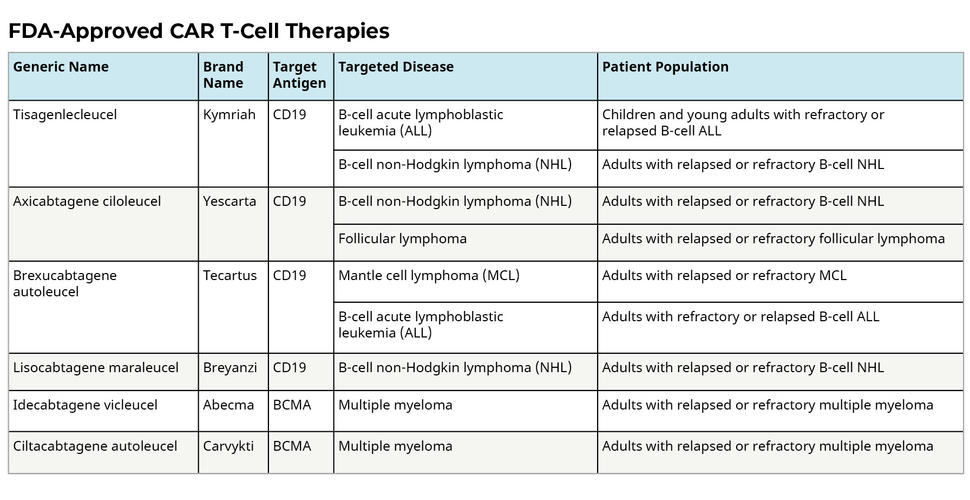
Meanwhile, the advent of CRISPR-based gene editing technologies offers tantalizing prospects for precise genomic manipulation in cancer treatment, with ongoing clinical trials exploring their potential across diverse malignancies.
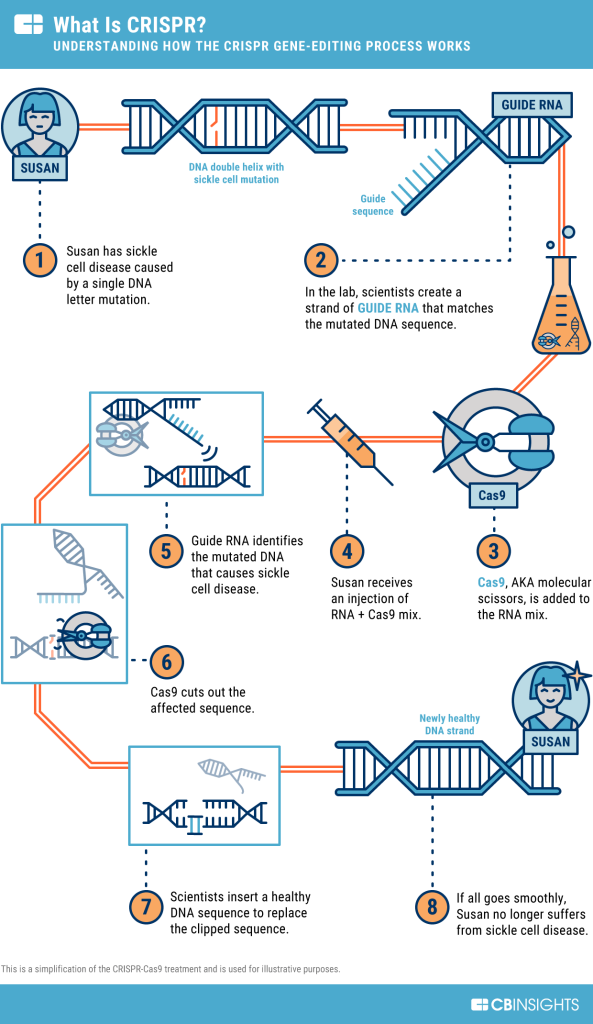
Charting the Course Ahead: Toward Hybrid Strategies and Technological Integration
Hybrid Warfare in Drug Development
The future landscape of cancer drug development envisages a hybrid approach, synergizing traditional cytotoxic agents with innovative targeted therapies and immunomodulatory interventions. Embracing tailored treatment regimens informed by individualized molecular profiling and microbiome analysis holds promise in optimizing therapeutic outcomes and mitigating adverse effects.
Harnessing Big Data and Artificial Intelligence
The integration of big data analytics and artificial intelligence (AI) stands poised to revolutionize drug discovery and development paradigms. Leveraging vast repositories of biomedical data, AI algorithms offer invaluable insights into target identification, drug design, and repurposing endeavors. However, challenges pertaining to data quality, regulatory compliance, and infrastructural investments underscore the imperative for judicious utilization of AI-driven methodologies in tandem with human expertise.
Embracing Human Creativity in the Age of Artificial Intelligence
The teamwork between human creativity and AI promises a future of joint innovation in cancer drug development. Although AI has huge potential in making drug discovery and treatment better, its success relies on working closely with human expertise. Even with advanced technology, the core of cancer drug development is still about reducing human suffering and facing the many challenges of this disease together.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts

Immunology & Oncology
The Silent Guardian: How GAS1 Shapes the Landscape of Metastatic Melanoma
GAS1’s discovery represents a beacon of hope in the fight against metastatic disease.

Immunology & Oncology
Resistance Mechanisms Unveiled: The Role of Glutathione S-Transferase in Cancer Therapy Failures
Understanding this dual role of GSTs as both protectors and accomplices to malignancies is central to tackling drug resistance.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.
Designing Better Sugar Stoppers: Engineering Selective α-Glucosidase Inhibitors via Fragment-Based Dynamic Chemistry
One of the most pressing challenges in anti-diabetic therapy is reducing the unpleasant and often debilitating gastrointestinal side effects that accompany α-amylase inhibition.














