The majority of the time, people today are only given an Alzheimer’s diagnosis after displaying well-known symptoms like memory loss. The best therapy choices at that stage only stop the symptoms from getting worse.
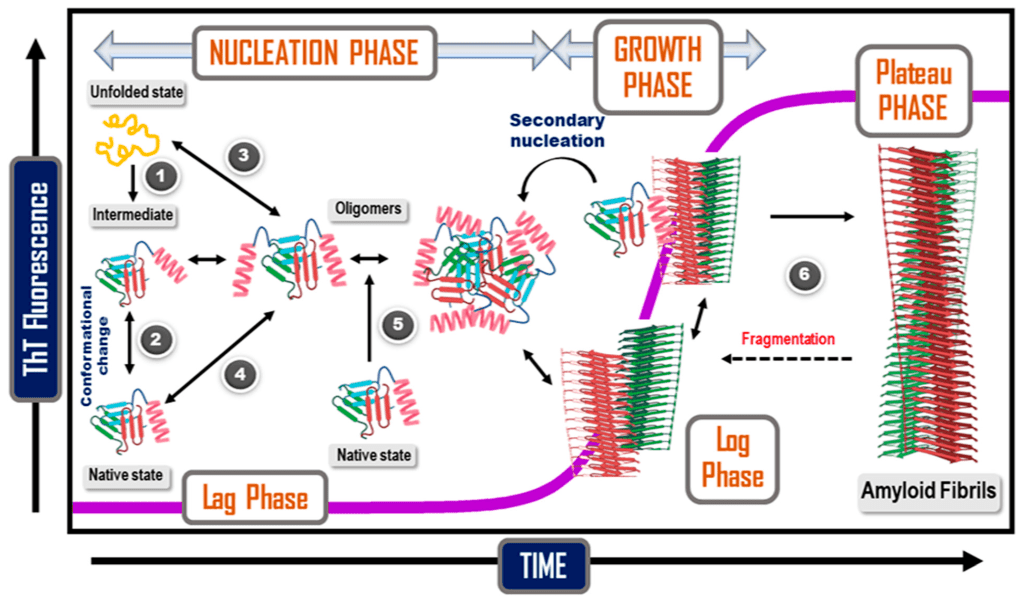
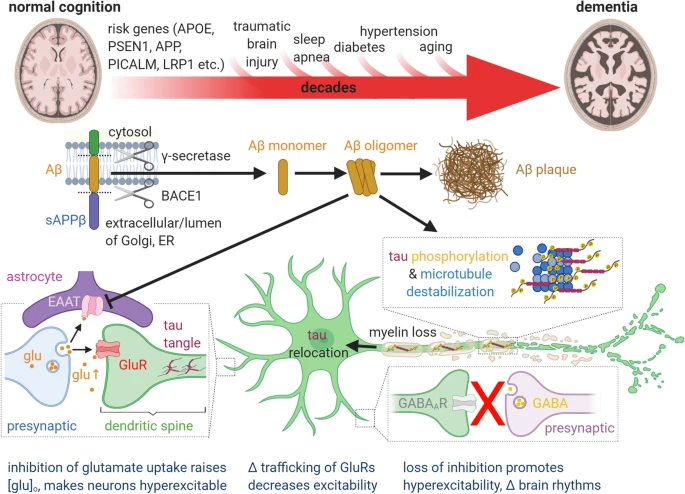
However, studies have shown that the precursors to Alzheimer’s disease begin to manifest years or even decades before symptoms that allow for a diagnosis to be made. These seeds are amyloid beta proteins, which cluster and misfold to form oligomers, which are tiny aggregates. These “toxic” oligomers of amyloid beta are believed to cause Alzheimer’s disease over time through a process that researchers are still trying to comprehend.
Alzheimer’s disease must be fought through early identification and disease-modifying therapies. Early diagnosis is a crucial first step in this process because it allows for intervention prior to irreversible damage, which is thought to start 10 to 20 years before symptoms appear. A potential target for the selective and early detection of these oligomers was made possible by the earlier identification of the α-sheet structure during conformational changes related to amyloidogenesis.
Not Nissin. Not Yaki. But SOBA.
A group of scientists at the University of Washington have created a lab test that can count the number of amyloid beta oligomers present in blood samples. Their test, known by the acronym SOBA which stands for soluble oligomer binding assay, could detect oligomers in the blood of patients with Alzheimer’s disease, but not in the majority of members of a control group who showed no signs of cognitive impairment at the time the blood samples were taken, they report in a paper published the week of December 5 in the Proceedings of the National Academy of Sciences.
Interestingly, 11 members of the control group had oligomers found in their blood by SOBA. 10 of these people had follow-up examination data, and all had minor cognitive impairment or brain pathology that was compatible with Alzheimer’s disease years later. In essence, for these 10 people, SOBA had identified the harmful oligomers prior to the onset of symptoms.
In addition to an assay that confirms an Alzheimer’s diagnosis, clinicians and researchers are looking for a reliable diagnostic test for the disease that can also identify early symptoms before cognitive decline occurs. This is crucial for both the general public’s health and all future studies into how harmful amyloid beta oligomers progress and do the harm they do. And according to senior author Dr. Valerie Daggett, a bioengineering professor at the University of Washington and a member of the faculty of the UW Molecular Engineering & Sciences Institute, SOBA might serve as the foundation for such a test.
The Biochemistry Behind
One of the early steps in the molecular pathogenesis of Alzheimer’s Disease (AD) is the development of toxic amyloid β-peptide (Aβ) oligomers. The estimated onset of these processes occurs 10 to 20 years prior to the onset of symptoms, and they include poor neural signaling, neuroinflammation, tau phosphorylation, and neurodegeneration. Toxic Aβ oligomers possess a nonstandard protein structure, termed α-sheet, and tailored α-sheet peptides target this main-chain structure in toxic oligomers regardless of sequence.
It has been determined from research results that the created α-sheet peptide suppresses harmful effects on neuronal signaling and also functions as a capture agent in our soluble oligomer binding assay (SOBA). Synthetic α-sheet-containing Aβ oligomers that have been pre-incubated generate robust SOBA signals, whereas monomeric and β-sheet protofibrillar Aβ do not. In contrast to a non-cognitively impaired control, cerebrospinal fluid (CSF) from an AD patient also included oligomers that contained α-sheet. The researchers created a plate coating to boost the density of the capture peptide for the detection of hazardous oligomers in plasma.
The toxic oligomers’ special characteristic is exploited by SOBA. A structure known as an alpha sheet is created when misfolded amyloid beta proteins start to group together into oligomers. Alpha sheets are uncommon in nature, and previous studies by Daggett’s team shown that they frequently bond to other alpha sheets. Her team’s creation of a synthetic alpha sheet that can bind to oligomers in blood or cerebrospinal fluid samples is the foundation of SOBA. The oligomers adhered to the test surface are verified to be made of amyloid beta proteins using then-standard procedures.
Testing, Detection and Assay Adaptation
The group examined SOBA using blood samples from 310 research participants who had previously consented to the use of their blood samples and some of their medical information for Alzheimer’s research. The patients had no history of dementia, Alzheimer’s disease, moderate cognitive impairment, or any other type of cognitive impairment at the time the blood samples had been taken.
Participants with mild cognitive decline and intermediate to extensive Alzheimer’s disease had oligomers found in their blood by SOBA. The diagnosis of Alzheimer’s disease in 53 cases was confirmed by autopsy after the research subject had passed away; toxic oligomers were found in 52 of their blood samples, which had been taken years earlier.
Records indicate that SOBA also found oligomers in control group participants who later experienced modest cognitive impairment. Blood samples from other control group members who didn’t suffer any impairment lacked hazardous oligomers.
Daggett’s team is working with scientists at AltPep, a UW spinout firm, to convert SOBA into a diagnostic test for oligomers. Daggett is also the Founder & CEO of AltPep. Together, they demonstrated how SOBA was easily adaptable to identify toxic oligomers of an additional class of protein known as α-synuclein oligomers linked to Parkinson’s disease and Lewy body dementia.
Prevention, Always Cheaper Than Cure
The study’s findings indicate that a number of human disorders are linked to the buildup of hazardous oligomers that result in these alpha sheet formations. Apparently, it extends beyond Alzheimer’s to include Parkinson’s, type 2 diabetes, and other diseases. That distinctive alpha sheet structure is being absorbed by SOBA. Therefore, it is hoped that this technology will help in the diagnosis and research of numerous other “protein misfolding” disorders.
In AD patients, including controls who later developed mild cognitive impairment, SOBA found Aβ oligomers. Additionally, SOBA was 99% sensitive and specific in identifying AD compared to other types of dementia, according to clinical and neuropathological diagnosis.
Daggett thinks the test has more upside. She anticipates that SOBA will help in the identification of those who are at risk or who are incubating the disease, as well as acting as a readout of therapy efficacy to aid in the development of early Alzheimer’s disease treatments.
Dylan Shea, PhD, from the UW Department of Bioengineering’s Molecular Engineering Program, is the study’s lead author. The Northwest Mental Illness Research, Education and Clinical Center, the Washington Research Foundation, and the National Institutes of Health all provided funding for the study.
Subscribe
to get our
LATEST NEWS
Related Posts
Chronic & Debilitating Diseases
Renopathology Tipping Point: Deciphering the Molecular Code of Stage 2 Chronic Kidney Disease
The molecular events of Stage 2 CKD, from inflammation to lipid metabolism, offer insights for diagnosis and treatment.
Chronic & Debilitating Diseases
A New Lens on Shock: Hemodynamic Insights Through Critical Care Ultrasound
CCU has transformed the hemodynamic assessment of shock, delivering a reliable, reproducible, and non-invasive tool for ICU clinicians.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.