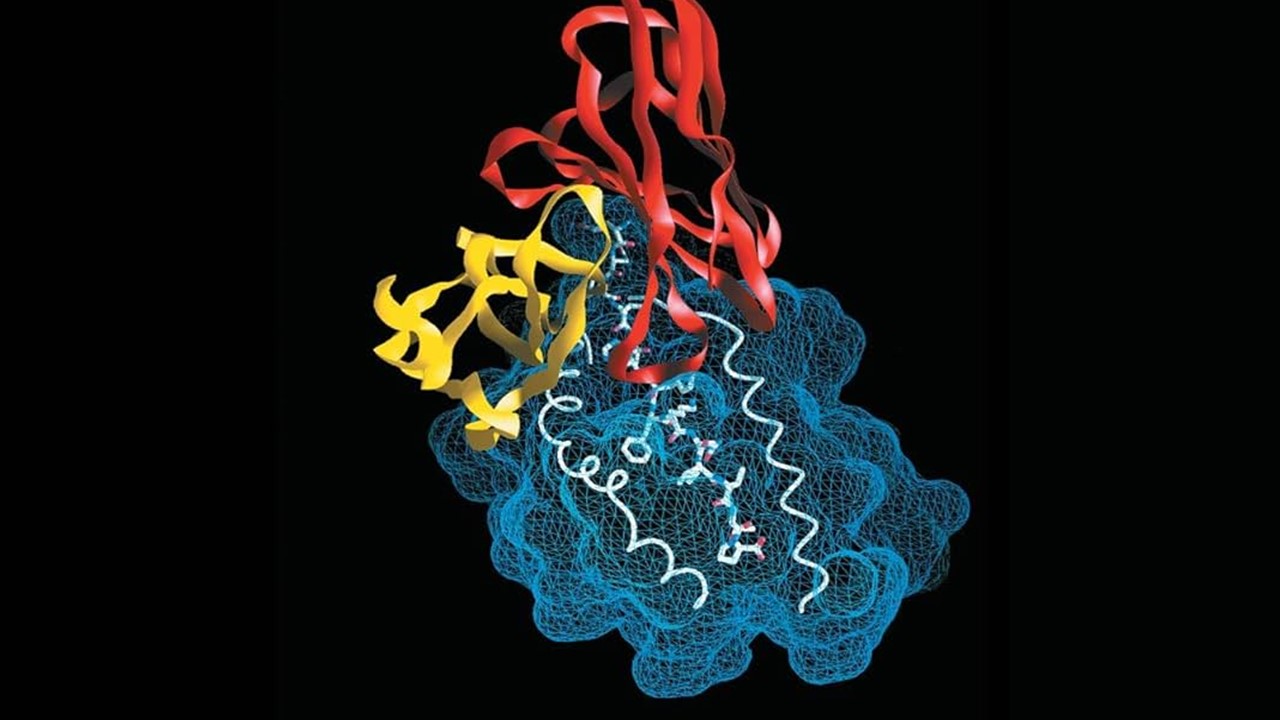
The development of drug delivery systems has evolved dramatically over the past few decades, with liposomal formulations taking center stage. These vesicles, known for encapsulating therapeutic agents, provide enhanced stability and reduce toxicity, offering a promising approach for delivering drugs to specific tissues or tumors. The progression from traditional drug discovery methods to modern lead discovery has been marked by significant advancements in targeting mechanisms, particularly with the development of active targeting techniques that optimize the delivery and effectiveness of liposomal drugs.
From Passive to Active Targeting: The Evolution of Liposomal Design
Early liposome formulations primarily relied on passive targeting, which leveraged the enhanced permeability and retention (EPR) effect observed in tumors. The EPR effect allowed liposomes to accumulate at tumor sites due to leaky vasculature, leading to higher drug concentrations in cancerous tissues than in healthy ones. While passive targeting improved therapeutic outcomes compared to conventional drug delivery, it was far from perfect. The advent of active targeting revolutionized liposomal drug delivery by modifying the liposome’s surface with targeting ligands that bind specifically to receptors on tumor cells.
Active targeting aims to increase the precision of drug delivery by attaching ligands to the liposomal surface that interact directly with receptors on target cells. Ligands such as antibodies, peptides, and nucleic acids are selectively chosen based on the tumor’s overexpression of certain receptors. This interaction not only directs the liposomes to the tumor cells but also facilitates the internalization of the drug through receptor-mediated endocytosis, enhancing intracellular drug delivery. By utilizing this approach, drug diffusion away from the tumor site is minimized, improving overall efficacy and potentially overcoming drug resistance observed in some cancers.
Selecting the Right Antigen: A Crucial Step in Targeted Liposomes
Choosing the appropriate target antigen is paramount to the success of targeted liposomal therapy. This selection process is meticulous, relying on factors such as the antigen’s overexpression on tumor cells and its minimal presence on healthy tissues. The degree of antigen expression plays a critical role, as demonstrated by HER2-targeted immunoliposomes, which have shown varying efficacy depending on receptor density on different breast cancer cells. For instance, SKBR-3 and BT-474 breast cancer cells, with their high HER2 receptor density, showed pronounced receptor-mediated endocytosis and cytotoxicity, while MCF-7 cells with low receptor density demonstrated reduced liposomal uptake and drug efficacy.
The balance between antigen overexpression and selective targeting is vital to avoid off-target effects and nonspecific toxicity. Moreover, the ability of the antigen to internalize the liposome upon binding is crucial. Studies have highlighted the importance of internalization, particularly for liposome-encapsulated drugs like doxorubicin, where internalizing ligands significantly enhanced antitumor efficacy compared to non-internalizing approaches. The degree of antigen shedding, especially in highly proliferative tumors, is another factor that must be considered. Excessive shedding can lead to the rapid clearance of liposomes from circulation, binding to soluble antigens, and reducing drug efficacy.
Engineering Targeted Liposomes: Overcoming Challenges in Design and Function
The design and development of targeted liposomes present several challenges, primarily revolving around the stability of the formulation and its pharmacokinetics. Liposomes must encapsulate the drug stably, circulate for extended periods, and release their contents only at the target site. Early iterations of immunoliposomes, where antibodies were covalently attached to the liposome surface, often resulted in accumulation in the liver, diminishing their therapeutic potential. The incorporation of PEGylation—a process that coats the liposomal surface with polyethylene glycol (PEG)—addressed many of these issues by enhancing liposome circulation time and reducing rapid clearance.
PEGylated liposomes can now carry targeting ligands attached via a PEG spacer arm, which extends beyond the liposome’s surface, reducing steric hindrance and improving receptor binding. This innovation allowed for more precise targeting and internalization. However, the design process is intricate, as there must be an optimal balance between ligand density and circulation time. Liposomes with high ligand density may experience rapid clearance due to interactions with immune cells, while those with insufficient ligand density may not bind effectively to their target.
The process of ligand attachment has also evolved, with several methods being employed to ensure stability and functionality. These methods include conjugating ligands to the phospholipid head groups of the liposome or using specialized PEGylated chains with functionalized ends that allow for covalent attachment of ligands. The post-insertion technique has proven to be particularly effective, enabling high-efficiency insertion of ligands into preformed liposomes while ensuring that all ligands are positioned on the outer surface for optimal interaction with target cells.
Antibody-Targeted Liposomes: A Case Study in Precision Medicine
One of the most extensively studied applications of targeted liposomal therapy is the use of antibody-targeted liposomes for treating cancer. Antibodies, particularly those of the IgG class, have been widely explored for their ability to selectively bind to tumor-associated antigens. These antibody-laden liposomes, or immunoliposomes, can be engineered to deliver chemotherapeutic agents directly to tumor cells, thereby increasing drug efficacy while minimizing damage to healthy tissues.
Antibody-targeted liposomes have demonstrated varying degrees of success. While some studies have shown increased tumor accumulation and improved therapeutic outcomes, others have reported no significant differences between targeted and non-targeted liposomes in terms of drug accumulation at tumor sites. This discrepancy has been attributed to several factors, including the clearance rate of targeted liposomes, receptor downregulation, and the binding site barrier effect, where the liposomes are restricted to the tumor’s periphery and fail to penetrate deeper into the tumor mass.
One promising approach has been the targeting of HER2-overexpressing tumors with anti-HER2 immunoliposomes. Studies have shown that while both targeted and non-targeted liposomes achieve high levels of tumor tissue accumulation, the targeted liposomes are more likely to internalize within tumor cells, leading to enhanced intracellular drug delivery and greater therapeutic efficacy.
Future Directions: Optimizing Ligand Density and Multivalency
As research into targeted liposomal therapy continues, a critical area of focus is the optimization of ligand density and multivalency. Recent studies have demonstrated that high-density, low-affinity ligands can provide better cellular uptake than higher-affinity ligands. This finding underscores the importance of multivalency, where multiple binding interactions between the liposome and the target cell enhance overall uptake and efficacy.
While the internalization of liposomes is often seen as a prerequisite for effective drug delivery, there are cases where non-internalizing liposomes have shown therapeutic benefits. For example, PEGylated liposomes loaded with vincristine or doxorubicin and modified with antibodies against non-internalizing antigens demonstrated significant efficacy, suggesting that drug release kinetics and tumor microenvironment play equally important roles in determining treatment outcomes.
In conclusion, the development of targeted liposomal therapeutics represents a significant leap forward in drug delivery systems. The ability to selectively direct drugs to tumor cells while minimizing off-target effects has opened new avenues for treating cancer and other diseases. However, the road to fully optimized liposomal formulations is still paved with challenges. Careful selection of antigens, precise engineering of liposome structures, and a deeper understanding of tumor biology are all necessary to maximize the therapeutic potential of this promising technology. As research progresses, the hope is that these advances will continue to translate into more effective and personalized treatments for patients.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.
Medicinal Chemistry & Pharmacology
Coprocessed for Compression: Reengineering Metformin Hydrochloride with Hydroxypropyl Cellulose via Coprecipitation for Direct Compression Enhancement
In manufacturing, minimizing granulation lines, drying tunnels, and multiple milling stages reduces equipment costs, process footprint, and energy consumption.

Medicinal Chemistry & Pharmacology
Decoding Molecular Libraries: Error-Resilient Sequencing Analysis and Multidimensional Pattern Recognition
tagFinder exemplifies the convergence of computational innovation and chemical biology, offering a robust framework to navigate the complexities of DNA-encoded science
Read More Articles
Magnetic Nanoengineering: Overcoming Biological Variability and Enhancing Therapeutic Precision
The future of nanomedicine lies in harmonizing precision, accessibility, and ecological responsibility, ushering in an era where therapies are tailored to individual biological landscapes.
Trials, Triumphs, and Trials Ahead: Navigating the Landscape of Randomized Controlled Trials in Artificial Intelligence-Driven Healthcare
The adoption of artificial intelligence in clinical practice has prompted a surge in randomized controlled trials, highlighting a balance of enthusiasm and prudence.
Illuminating the Dark Genome: Uncharted Frontiers in Therapeutic Discovery
The dark genome is not a biological void but a frontier awaiting illumination.