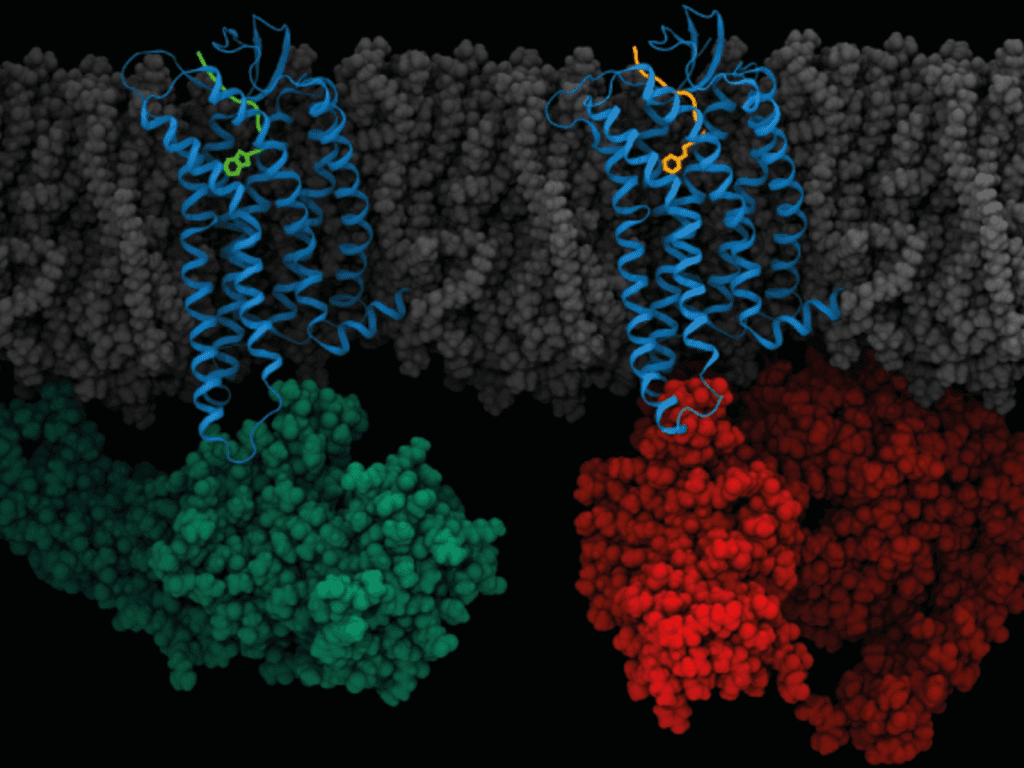
G-protein coupled receptors (GPCR) belong to one of the largest and most diverse groups of receptors to exist in the human body. These receptors play a critical role in signalling pathways which mediate some of the most important physiological processes, including neurotransmission and hormone signaling. In recent years, GPCR-targeted drug discovery has demonstrated the potential for exciting therapeutic innovations across areas like oncology, utilising biotechnological advancements such as nanobody tethering.
Introduction
GPCRs are the largest family of membrane proteins and are critical for a host of physiological processes, by mediating extracellular signalling with molecules like hormones, ions, and neurotransmitters. They consist of a single polypeptide chain that passes through the plasma membrane of the cell 7 times. In terms of a mechanism of action, they work by interacting with a molecule known as a trimeric G-protein, which is activated when the compound GTP is bound to the alpha subunit (of the G-protein).
These types of receptors are incredibly important for optimal physiological function, especially the nervous system which utilises GPCRs to facilitate neurotransmission. However, their ability to modulate such an immense range of physiological signals, makes the body vulnerable to a host of issues, especially if these receptors undergo damage or mutation.
Mutations in 55 GPCR genes are known to cause around 66 inherited monogenic diseases in humans. Hyperthyroidism and carcinomas are prime examples, in addition to the impairment of bone development or metabolism, resulting in a number of osteopathologies.
Their involvement within a variety of critical processes like the immune response and role in cellular communication, have made them a target for a huge number of currently prescribed drugs. According to a 2019 Nature publication, they comprise the most important class of drug targets, accounting for 12% of all human protein drug targets and the therapeutic effects of approximately 34% of clinically used drugs.
GPCR-targeting nanobodies
Small molecules make up approximately 80% of the FDA–approved GPCR-targeting drugs. However, obtaining small molecule drugs with high affinity, potency and selectivity plays a major challenge in the drug discovery process.
Another challenge is the complexity and highly dynamic nature of GPCRs, which also poses a significant challenge for drug discovery of small molecule candidates. To resolve some of these challenges, many pharma companies and biotechs have been investigating the viability of biologics, over small molecule drugs targeting GPCRs.
In recent years, researchers have identified that GPCRs can be targeted by biologicals, including antibodies and antibody fragments, but more specifically, nanobodies. A nanobody is essentially a type of miniature, engineered antibody which is derived from a heavy-chain only subset of (camelid) immunoglobulins.
The small molecular size, and stability of nanobodies, has made them a desirable drug delivery system for a number of therapeutic applications. This includes the topical delivery of drugs to the airways of patients infected with COVID-19.
The therapeutic use of antibodies and their derivatives demonstrate a number of advantages over small molecules: (1) high affinity and specificity (2) less variability in pharmacokinetics between patients (3) less frequent dosing.
Nevertheless, antibody therapeutics present some challenges of their own, including high production costs, limited tissue penetration and the inability to target intracellular targets.
Despite these hurdles, researchers have been investigating the viability of nanobodies for detecting, stabilising, modulating and therapeutically targeting GPCRs. In addition to their small size, their protruding antigen binding loops have been noted as favourable features, for developing selective and potent GPCR-binding molecules.
Latest research
Research is ongoing in this area to optimise the targeting of GPCRs via nanobodies. A 2020 study developed a new type of conjugate that consists of a nanobody and peptide (ligand) fused together for a GPCR target.
Antibody drug conjugates (ADCs) are complex molecules used in cancer research to deliver chemotherapy agents. They are typically defined as antibodies to which other molecules are bound, through a chemical linker. While the success of ADCs have been evident across a number of indications, far fewer studies have assessed the viability of antibodies to deliver peptides (ligands) to the surface of receptors such as GPCRs.
The 2020 Nature study demonstrated that the conjugation of the peptide ligand to a nanobody substantially increased the agonist potency and receptor selectivity at the GPCR target, than the peptide alone. This highlights an exciting discovery for targeting GPCRs, by utilising the therapeutic advantages of nanobodies to support better ligand-receptor interactions.
Challenges in targeting GPCRs
There are, of course, a number of challenges with GPCR-targeted drug discovery, most of them focusing around specificity. One example is the binding site of the receptor M1,which has shown to be a well-validated target for alleviating cognitive decline in neurodegeneration. However, this binding site is virtually identical to those of the related receptors M2, M3, M4, and M5 – hence this gives rise to dose-limiting side effects like cardiovascular problems.
Another potential source of side effects can arise when targeting receptors that are implicated in multiple signalling pathways. Therefore, the development of GPCR-targeting drugs has been focused on improved specificity and efficacy in validated targets.
Targeting GPCRs in cancer
Research in molecular genetics has identified key GPCRs, whose mutations or altered expressions are linked with cells capable of producing tumours. GPCR-targeted therapy is a relatively novel area of anticancer drug development with significant potential, but challenges that also need to be addressed.
It has been shown that tumour cells can hijack GPCRs and cause them to over-express and become activated by releasing agonists into the extracellular environment. However, even in the absence of agonists, GPCRs can become tumorigenic if the receptor has undergone genetic mutation. Therefore, researchers have been focused on understanding the activation mechanism of GPCRs, and how this can be exploited in anti-cancer drug development.
The approach for GPCR-targeted drug discovery in oncology is focused on the signalling of the receptors using agonists or antagonists, and targeting the specific receptor-ligand interactions. This mechanism can then be used to transport anti-neoplastic drugs or toxins to cancerous cells.
Despite the incredible potential of GPCR-targeted anti-cancer drugs, only eight anti-cancer drugs targeting GPCRs received FDA approval (up until 2018), including degarelix for prostate cancer and lanreotide for pancreatic cancer. However, more accurate investigations into the biological mechanism linking disease pathology and GPCR implication, will no doubt yield more promising results in drug discovery for cancer, and hopefully other therapeutic areas too.
Charlotte Di Salvo, Editor & Lead Medical Writer
PharmaFeatures
Subscribe
to get our
LATEST NEWS
Related Posts

Drug Discovery Biology
Proteolytic Rewriting: Engineering Controlled Absence of Pathogenic Protein Persistence
Targeted protein degradation transforms drug therapy by engineering the cellular machinery to erase, rather than merely inhibit, pathogenic proteins.

Drug Discovery Biology
Open Nucleotides: Grant-Driven Infrastructure for Equitable mRNA Vaccine Manufacturing
By developing accessible cap analogs and RNA raw materials, Hongene Biotech, guided by David Butler’s expertise in nucleotide chemistry and supported by the Gates Foundation, is reshaping the molecular infrastructure that underpins global mRNA vaccine equity.
Drug Discovery Biology
Redox Messengers: How Endogenous Gasotransmitters Rewire Vascular Biology to Resist Age-Driven Oxidative Stress
Gasotransmitters provide a biologically sophisticated means of counteracting age-related oxidative stress and preserving vascular resilience.
Drug Discovery Biology
Aptamer Signal Dynamics: Engineering Nucleic Acid Recognition Systems for High-Fidelity, Multi-Modal Biosensing
Aptamers redefine biosensing by pairing programmable molecular recognition with versatile transduction strategies capable of detecting clinically relevant biomarkers with exceptional fidelity.
Read More Articles
Spatial Collapse: Pharmacologic Degradation of PDEδ to Disrupt Oncogenic KRAS Membrane Localization
PDEδ degradation disrupts KRAS membrane localization to collapse oncogenic signaling through spatial pharmacology rather than direct enzymatic inhibition.
Neumedics’ Integrated Innovation Model: Dr. Mark Nelson on Translating Drug Discovery into API Synthesis
Dr. Mark Nelson of Neumedics outlines how integrating medicinal chemistry with scalable API synthesis from the earliest design stages defines the next evolution of pharmaceutical development.
Zentalis Pharmaceuticals’ Clinical Strategy Architecture: Dr. Stalder on Data Foresight and Oncology Execution
Dr. Joseph Stalder of Zentalis Pharmaceuticals examines how predictive data integration and disciplined program governance are redefining the future of late-stage oncology development.
Exelixis Clinical Bioanalysis Leadership, Translational DMPK Craft, and the Kirkovsky Playbook
Senior Director Dr. Leo Kirkovsky brings a rare cross-modality perspective—spanning physical organic chemistry, clinical assay leadership, and ADC bioanalysis—to show how ADME mastery becomes the decision engine that turns complex drug systems into scalable oncology development programs.
Policy Ignition: How Institutional Experiments Become Durable Global Evidence for Pharmaceutical Access
Global pharmaceutical access improves when IP, payment, and real-world evidence systems are engineered as interoperable feedback loops rather than isolated reforms.
Sepsis Shadow: Machine-Learning Risk Mapping for Stroke Patients with Bloodstream Infection
Regularized models like LASSO can identify an interpretable risk signature for stroke patients with bloodstream infection, enabling targeted, physiology-aligned clinical management.







