About the Interviewee (Sourced from Parthenon Therapeutics’ Official Website)
Laurent Audoly, PhD is Co-Founder, CEO & Chairman of Parthenon Therapeutics.
He has contributed to the identification and development of seven novel medicines (Xeljanz®, Hemangiol®, Javlor®, Renflexis®, Brenzys®, Ontruzant®, Hadlima® – biologics and small molecules) addressing unmet medical need across multiple disease areas that generate > $3B in annual sales.
Laurent has also led business and R&D functions in both pharma and biotech (US and EU), including CEO of Kymera Therapeutics, head of R&D with Pierre Fabre Pharmaceuticals, and positions of increasing leadership responsibilities on the business and science fronts at Pfizer, Merck, and Pieris contributing to the advancement of 21 drug candidates into clinical development across multiple disease areas and modalities. He has completed partnering transactions worth over $3B in biobucks and raised > $250M.
He studied chemistry and pharmacology (Ph.D.) at Vanderbilt University and completed postdoctoral training at University of North Carolina – Chapel Hill and Duke University where he was the recipient of an American Heart Association Fellowship. He has co-authored over 70 papers and patents. Laurent also serves as an independent director on the board of Cytovia, Ixaka, Ermium, and is a member of Ventus’ scientific advisory board.
The Discussion
The Parthenon Ethos and Dr. Audoly’s Motivations
[Dex Marco]: It’s such a pleasure and privilege to have you here with us today, Dr. Laurent. You have a rather long, undeniably impressive background as a serial biotech entrepreneur and senior executive in the industry – what would you say drove you towards this field, and what brought you to co-found Parthenon Therapeutics?
[Dr. Laurent]: The pursuit of cancer research is highly relevant and greatly impactful. Cancer affects not only the patient but also the patient’s family. It is a rewarding experience to be able to play a part in the elucidation of the mechanisms by which cancer operates on a multi-faceted level because it allows scientists to develop medications that are not only sustainably effective but also of reduced side effects. And so, we are aiming for a patient-centric approach at Parthenon as we gear towards precision medicine.
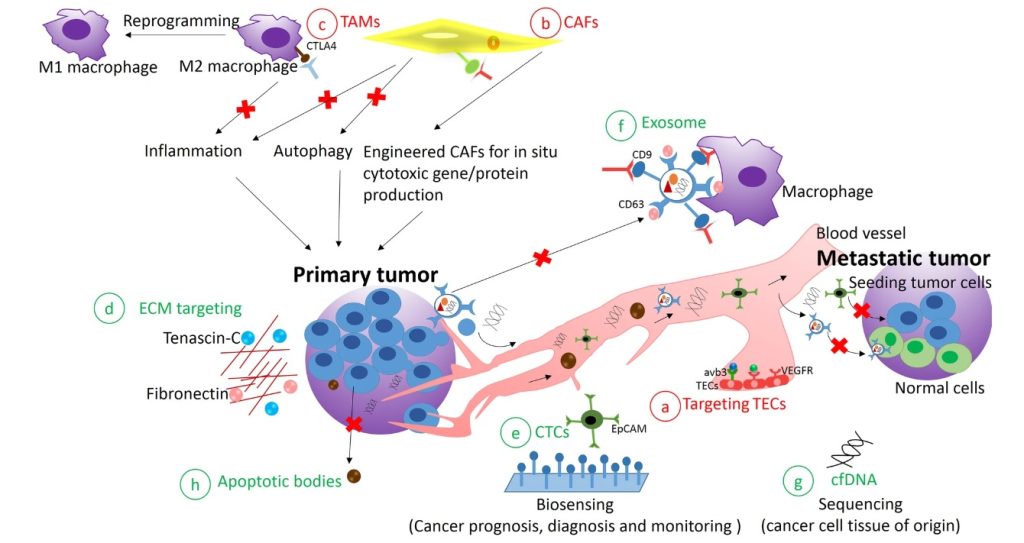
Currently, the tumor microenvironment (TME) is the focus of cancer metastasis and growth research.
A lot of appreciation must be given to our current understanding of the TME – the healthy blood arteries, chemicals, and cells that surround and supply a tumor cell. A tumor can change its microenvironment, and the microenvironment can affect how a tumor grows and spreads. Because of the TME’s complexity and diversity, which includes a number of components and characteristics that are closely related to the occurrence and development of cancer, targeted regulation of TME components or signaling pathways has emerged as the key to preventing the spread and invasion of cancer.
In addition, we’re also looking to revolutionize cancer treatment using immune checkpoint inhibitors while also spotting mechanisms of resistance.
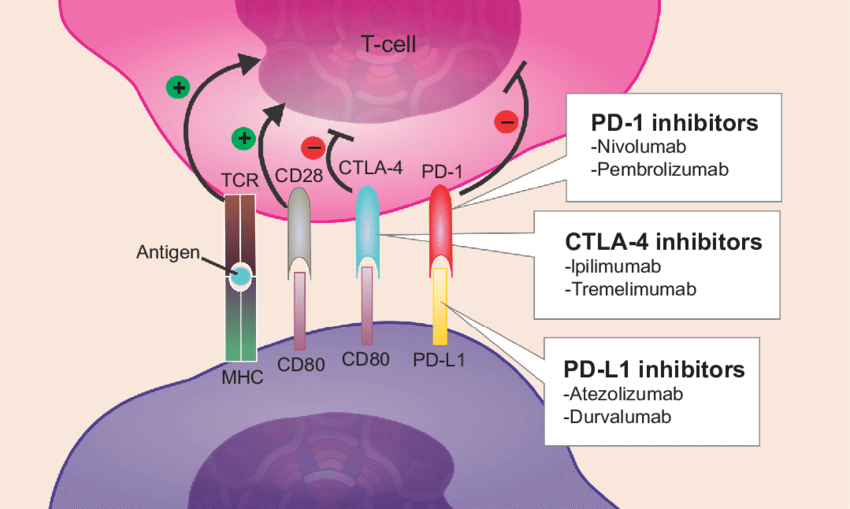
Immune cells known as T cells are potent cancer-fighting tools used by the immune system. Cell surface immunological checkpoints aid in regulating an immune response.
Immune checkpoints typically keep T cells dormant, or in a “off” state, until they are required. Thus, the T cells are prevented from damaging healthy cells. These checkpoints can be exploited by cancer cells to deactivate T lymphocytes. This prevents the death of the cancer cells. Drugs known as immune checkpoint inhibitors stop the checkpoints. The T cells can now assault the tumor as a result. Some forms of cancer can be effectively treated with immune checkpoint inhibitors, but they are not effective in all patients and can have harmful side effects.
These are the core strategies that we implement as we break barriers to better cancer treatment. At Parthenon Therapeutics, we are reprogramming the tumor microenvironment to beat cancers.
The Collaborative Process of Drug Discovery & Development
[Dex Marco]: I would like to highlight and commend how you were pivotal in the identification and development of seven novel medicines addressing unmet medical needs across multiple disease areas that generate more than $2 Billion in annual sales. This is not an easy feat, working across an array of drug development studies. And not only that, you’ve also ensured both their clinical and market successes. How were you able to manage collaboration overload?
[Dr. Laurent]: It’s gracious of you to highlight the part I played in the development of these medications but in reality, drug discovery and development is a team sport. It took hundreds and thousands of brilliant minds, experienced researchers and effective technical, process, legal and business management to push through the development of a single lead compound with observed bioactivity thru the pre-clinical and clinical trials until it reached the market. The drug development business requires a very large team effort with so many other stakeholders. It is a wonderful opportunity to put in place a special collaboration. I am personally privileged to meet a ton of incredible human beings as I’ve been in the industry longer than I could imagine.
With all these inspiring people surrounding you, you gotta go all in!
Despite busy times and unrelenting deadlines, it is a hugely rewarding experience to be an “enabler of a much bigger process”.
I am a drug developer and when I see an opportunity for a novel mechanism to target, I go grab it and run it with biology, chemistry and our current bleeding-edge knowledge of the disease process in order to make better medicines that are not only effective but are also, at best, mute of untoward effects. However, this is not easy, because as we dive deeper, we get to see ourselves knowing a lot less. Nevertheless, we continue to strive as we search better understanding of how mechanisms of disease work and how we can remedy them.
And it’s always about picking the right people, creating highly focused and dedicated teams from these chosen ones and accepting the beauty that is teamwork for it is a noble pursuit to be part of a greater and more improved drug development process and more.
Precision Oncology: An Expected Slow Uptake Despite Fast Science
[Dex Marco]: Accenture’s study with 130 oncologists from the US and Europe revealed that despite the promise of precision oncology, adoption is slow. In addition, more than 80% of oncologists believe precision oncology is important, yet most don’t fulfill the critical elements for adoption in clinical practice. Why do you think this is the case?
[Dr. Laurent]: Science nowadays moves pretty quickly. We are in the most fortunate of times where data is readily available for sharing and we have the digitalization to thank that for. This has allowed improved collaboration globally and is the very reason why scientific progress is expedited. Nevertheless, I don’t find it surprising that the uptake of such novel technologies and tools, such as precision oncology, is slow.
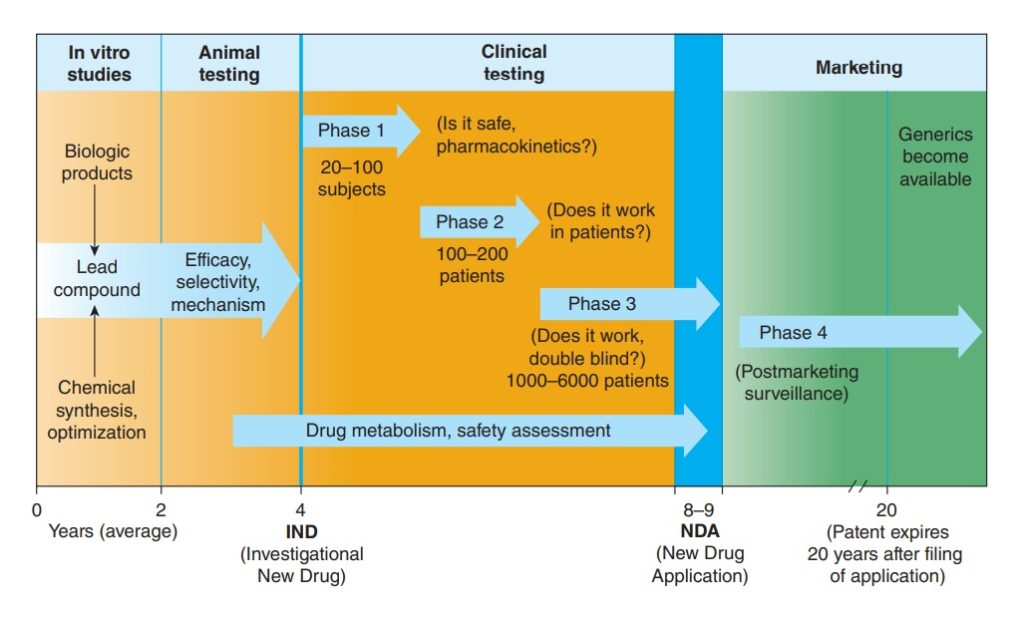
What happens in the laboratory is different with what happens in the clinic. And what happens in the clinic will have significant differences with what we can expect when drugs are already released in the market.
The reason for this is translatability. Laboratory experiments have control groups and sample sizes are expected to be smaller because we’re initially dealing with proof-of-concept and verifying biological activity and relevant properties of the chemical compound that shows promise of being a drug candidate. Also, there are no human subjects involved in pre-clinical trials. When it passes through tests and gets into the clinical trials, although there is an increase in sample size and we’re actually implementing proper dose-response observations for pharmacokinetic profiling and side effects monitoring, we still do not have the global population as our sample size and that means a lot of unseen and untoward side effects cannot be observed simply because, first, it’s not cost efficient and second, there’s too much genetics at play, hence the need for precision medicine for personalized treatments.
Nevertheless, we all proceed with precision and accuracy with the science that we know. Because, although the multifacetedness and complexities of these differing phases where these medications have gone through for testing and validation of efficacy in a way stifles full translatability because of inevitable variations in parametric control, we are all seeing movement. We can see oncology moving in the right direction with precision medicine, pharmacologic targeting of the tumor microenvironment, identification of mechanisms of resistance of checkpoint inhibitors, and multi-omics, among others.
Addressing Precision Oncology Needs The Parthenon Way
[Dex Marco]: It cannot be overstated that the future of precision oncology hinges on adoption. Oncologists say the need (1) data, (2) clinical decision support, and (3) evolved precision oncology education. As a precision oncology biotechnology company, how does Parthenon Therapeutics address these needs?
[Dr. Laurent]: At Parthenon Therapeutics, I can say that we can easily address the first two needs (data and clinical decision support) of oncologists as precision oncology vies for adoption. Only real-world data sets with high-quality underlying data can be considered credible. Clinical decisions are better supported by more data when there is more data.
I have to really emphasize that this endeavor requires a deep connection with the science as we apply it in the laboratory, into and through the clinic, expedited by established partnerships and even until it reaches marketing and commercialization on a global scale. Everything has to be backed by data. In that way, stakeholders are shown to be scientific, methodical, reliable, and excellent. In turn, the data generated, sorted, cleaned and transformed into information will be as equally as scientifically based, methodically produced, reliably distributed, and excellently translated into the needed deliverables.
In this industry you have to be able to explain the science, believe that the science works, generate verifiable data from the experiments, believe in the data, gather the needed people for your team, and believe in your team’s capacity to assess, address, and to impress.
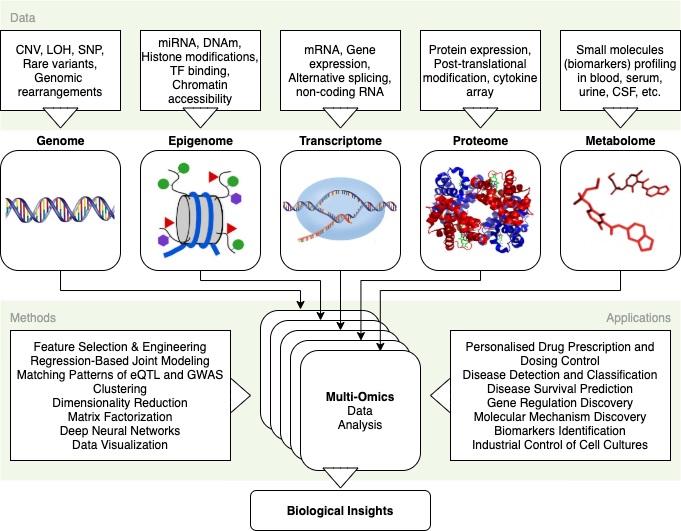
Multi-omics: The Big Picture Approach
[Dex Marco]: In recent years, next-generation sequencing has become better at identifying both the mutations in DNA and the changes in gene expression and post-translational modifications. Multi-omics data are offering the opportunity to redefine the precision oncology paradigm. What are the main advantages of using this bigger picture approach and what challenges can we expect with its implementation?
[Dr. Laurent]: I want you to picture an aquarium. And as you observe this aquarium on a distance, you’ll see the fishes inside it from that perspective. Now assign this as one type of an omics discipline in biology, say genomics. So, there’s observational data obtained from this side of the aquarium. However, in cancer, as in all other disease states, there is an undeniable need for holistic molecular measurements to better understand disease initiation, development, diagnosis, and therapy. And relying on one type of analysis, as in the case of genomics, won’t be sufficient to generate an all-encompassing exhaustive database where, at best, every biomarker is identified, one can directly measure causes and consequences of biological phenotypes, rather than make inferences from incomplete data, genotype-phenotype matching is improved, and molecular mechanisms of mutation, signaling or upregulation/downregulation and pinpointing of target proteins established.
This is where the other omics come in.
We have epigenomics, microbiomics, lipidomics, proteomics, glycomics, foodomics, transcriptomics, metabolomics, nutrigenomics, pharmacogenomics, pharmacomicrobiomics, toxigenomics, and miscellaneous ones like pscychogenomics, connectomics, cellomics, tomomics and stem cell genomics. Now imagine all these omics disciplines to be the other angles with which you can view the aquarium. With all the data obtained from these omics disciplines, we can then employ advanced mathematical and computational techniques to generate maps, schemes, representations and other useful parameters that improve our understanding of cancer biology and cancer treatment. In short, A multi-omic approach increases our understanding of biology by helping us see things that would be hidden with one type of data.
In order to comprehend and model the behavior of cancer cells in a predictive manner, the community of researchers studying cancer is increasingly embracing this complexity. Systems biology methods are assisting in the understanding of the mechanisms underlying tumor progression and the development of more potent cancer treatments. Together, these methods and the quickening pace of technological development allow for the most thorough, accurate, and high-throughput data collecting ever. These data enable computational and mathematical models to build predictive biomarkers, pinpoint critical unregulated systems and processes, and enhance therapy approaches. Future targeted therapies will be much more specific and effective thanks to the implementation of patient-specific computational and mathematical models of cancer, which will also hasten the acceptance of personalized and precision cancer medicine.
Although multi-omic evaluations provide a wealth of information, doing so necessitates analyzing and interpreting exceedingly huge data sets, which reduces analytical throughput and adoption ease. The most promising approach to overcoming these difficulties uses computational approaches leveraging artificial intelligence (AI), however despite the conceptual advantages of AI and its successful usage in single-omic studies, the use of AI in multi-omic studies is still relatively uncommon. This is one of the major challenges that we face in implementing multi-omic strategies in cancer research.
The Future of Oncology & Hopes of Dr. Audoly
[Dex Marco]: What other impacts do you hope to have on the industry as the CEO of Parthenon Therapeutics, especially in context of the vision for Precision Medicine Approach in Oncology strategy and operations?
[Dr. Laurent]: A lot of experienced, trained and knowledgeable people working together can do amazing things. I’ve seen that magic happen at Vanderbilt [University] and Northeastern [University].
If I have one hope, not just in the pursuit and furtherance of Precision Medicine in Oncology, but in the scientific research community in general, it’s that we need to train the next generation of talent. Some simple objectives come into mind like, ‘How do we make a difference?’, and ‘How do we ensure that our sector has access to the best possible talent?’. Drug Discovery and Development is a people business. And I’ve seen that firsthand as great strides have flourished at Vanderbilt University, where I took my PhD, and at the Institute of Experiential Artificial Intelligence (EAI) at Northeastern University, where a platform that can impact approaches across R&D in life sciences, drug discovery, automation, -omics-driven hypotheses, systems-based biology, and the translation to patients, to name a few, is currently being built.
In hopes of better understanding the molecular biology of the disease and finding the most effective way to cure that dysfunction, we need to accept the multidimensionality of this endeavor and think ‘How do we establish training programs for post-docs and other talents so that not only technical precision and accuracy can be fostered but so does creativity, dedication and the passion to continue what has been started by the hundreds and thousands of scientists before us?’
Discovery is one thing but understanding the biology of a disease using quantitative sciences is another. It is vital, nowadays and in the years to come, to describe biology using math. In addition to having top-shelf science, I want to see good experimentalists running proper controls by being able to also describe their work very effectively and if they’re interested, ‘How do you fund all that great work?’, ‘How do you get deals done?’, ‘How do you work with other people?’, ‘How do you collaborate?’, et cetera. So, it’s both science and math, but also people skills, negotiation skills, so on and so forth. So, I think that’s also one of the key opportunities in our sector is to go beyond sort of the deliverables themselves, but to ask ourselves, ‘Where are the other pillars that underpin the success of our sector?’
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES
Join Proventa International’s Oncology Strategy Meeting in Boston this November to hear more precision oncology, multi-omics, the tumor microenvironment, and cell-based therapeutics from leading experts in the field. Click on the photo below to download the brochure and register for the event.

Web Media Acknowledgements
(1) Title Background Featured Banner Image of Dr. Laurent Audoly is adapted from Sophie Arutunian’s article entitled Santé : à Toulouse, les Laboratoires Pierre Fabre tendent la main aux startups. Photograph is taken by Rémi Benoit. Article was published on 05 Oct 2015, 14:25. The image was retrieved on November 8, 2022 from https://toulouse.latribune.fr/decideurs/entreprises/2015-10-05/sante-a-toulouse-les-laboratoires-pierre-fabre-tendent-la-main-aux-startups.html.
(2) Tumor Microenvironment Pharmacologic and Diagnostic Targets image used in this article is adapted from Fig. 2 in the Cell Communication and Signaling open access journal entitled Tumor microenvironment complexity and therapeutic implications at a glance. The article was authored by Roghayyeh Baghban, Leila Roshangar, Rana Jahanban-Esfahlan, Khaled Seidi, Abbas Ebrahimi-Kalan, Mehdi Jaymand, Saeed Kolahian, Tahereh Javaheri & Peyman Zare. Article was published on 07 Apr 2020. The image was retrieved on November 8, 2022 from https://biosignaling.biomedcentral.com/articles/10.1186/s12964-020-0530-4/figures/2.
(3) Mechanism of Action of Immune Checkpoint Inhibitors image used in this article is adapted from Fig. 2 in the OncoTargets and Therapy peer-reviewed medical journal entitled Potential role of immunotherapy in advanced non-small-cell lung cancer. The article was authored by Ramon Andrade De Mello, Ana Flávia Veloso, Paulo Esrom Catarina, Sara Nadine, and Georgios Antoniou. Article was published on 16 Dec 2016. The image was retrieved on November 8, 2022 from https://www.researchgate.net/publication/311968982/figure/fig2/AS:449724315639811@1484234040453/Mechanism-of-action-of-immune-checkpoint-inhibitors-Notes-T-regs-depend-on-the-activity.png.
(4) Muti-omics image used in this article is adapted from a Medium article entitled Multi-Omics Data Factor Analysis. The article was authored by Oleksandr (aka Alex) Gurbych. Article was published on 5 April 2021. All rights of the article and the photograph belong to Oleksandr (aka Alex) Gurbych. The image was retrieved on November 8, 2022 from https://miro.medium.com/max/720/0*hoc3CvHaql0e_q1v.png.
(5) Precision Medicine in Oncology image used in this article is adapted from the Official Website of the National Research Council (CNR) Institute for Genetic and Biomedical Research (IRGB). The image was retrieved on November 8, 2022 from https://irgb.cnr.it/projects/precision-medicine-in-oncology/.
Print Media Acknowledgements
(1) The image on the development and testing process required to bring a drug to market in the USA shown above is adapted from Fig. 1-6 in Chapter 1 of Basic and Clinical Pharmacology, 14th Edition Lange medical book reference entitled Introduction: The Nature of Drugs & Drug Development & Regulation. The book chapter was authored by Bertram G. Katzung, MD, PhD with contributions from Barry Berkowitz, PhD. The book is copyrighted © 2018 by McGraw-Hill Education. The image was retrieved on November 8, 2022 via a digital copy of the aforementioned reference.
Subscribe
to get our
LATEST NEWS
Related Posts

Interviews
Financing the Future of Health: Navigating Biotech Investment Risks and Rewards with David Crean, Cardiff Advisory LLC & 1004 Venture Partners
A deep dive into how strategic biotech investments balance scientific innovation with financial foresight in an era redefining the economics of health.

Interviews
The Economics of Innovation: Optimizing Clinical Trial Investments with Robert Gabriel, Ashur Capital
A thought-provoking discussion on aligning scientific rigor, venture strategy, and financial intelligence to maximize R&D efficiency and accelerate the path from discovery to market.

Interviews
Redefining Evidence Generation: Integrating eCOA Science into Biotech Development with Saima Khakwani, Clario
Saima Khakwani shares how biotechs can accelerate development and improve data quality through robust eCOA science, meaningful endpoints, and patient-centric digital measurement.

Interviews
Clinical Research 4.0: Bridging Global Standards with Local Realities and Expanding Patient Access with Rania Alshami, PDC CRO
Rania Alshami leads PDC-CRO in advancing clinical research across the Middle East and Africa by integrating global scientific standards with regional realities to accelerate patient access to innovative therapies.
Read More Articles
Spatial Collapse: Pharmacologic Degradation of PDEδ to Disrupt Oncogenic KRAS Membrane Localization
PDEδ degradation disrupts KRAS membrane localization to collapse oncogenic signaling through spatial pharmacology rather than direct enzymatic inhibition.
Neumedics’ Integrated Innovation Model: Dr. Mark Nelson on Translating Drug Discovery into API Synthesis
Dr. Mark Nelson of Neumedics outlines how integrating medicinal chemistry with scalable API synthesis from the earliest design stages defines the next evolution of pharmaceutical development.
Zentalis Pharmaceuticals’ Clinical Strategy Architecture: Dr. Stalder on Data Foresight and Oncology Execution
Dr. Joseph Stalder of Zentalis Pharmaceuticals examines how predictive data integration and disciplined program governance are redefining the future of late-stage oncology development.
Exelixis Clinical Bioanalysis Leadership, Translational DMPK Craft, and the Kirkovsky Playbook
Senior Director Dr. Leo Kirkovsky brings a rare cross-modality perspective—spanning physical organic chemistry, clinical assay leadership, and ADC bioanalysis—to show how ADME mastery becomes the decision engine that turns complex drug systems into scalable oncology development programs.
Policy Ignition: How Institutional Experiments Become Durable Global Evidence for Pharmaceutical Access
Global pharmaceutical access improves when IP, payment, and real-world evidence systems are engineered as interoperable feedback loops rather than isolated reforms.