The next frontier in precision medicine lies in drugs that surgically disrupt disease-specific metabolic pathways and recalcitrant enzymes—compounds designed not merely to block symptoms but to reprogram the biochemical infrastructure of pathology itself. Unlike traditional drugs that target surface receptors or generic cellular processes, these metabolic interventions strike at the heart of disease mechanisms by hijacking the unique biochemical dependencies of pathological cells. From small molecules that starve tumors of oncometabolites to covalent inhibitors that permanently disable rogue kinases, this new generation of drug candidates operates with the precision of molecular locksmiths. The implications span oncology, neurodegeneration, and infectious diseases—each field now developing compounds that exploit metabolic vulnerabilities invisible to conventional pharmacology.
Oncometabolite Addiction: Exploiting Cancer’s Metabolic Achilles’ Heels
Cancer cells rewire their metabolic circuitry to fuel uncontrolled proliferation, creating dependencies that modern drug candidates ruthlessly exploit. Tumor biologists have identified numerous malignancies addicted to specific metabolites—like IDH-mutant gliomas relying on 2-hydroxyglutarate or triple-negative breast cancers dependent on serine biosynthesis. These addictions emerge because cancer cells often mutate themselves into metabolic corners where alternative pathways can’t compensate for inhibited ones.
IDH inhibitors exemplify this strategy. Drugs like ivosidenib block mutant isocitrate dehydrogenase enzymes from producing the oncometabolite R-2-hydroxyglutarate, effectively starving tumors of a molecule they require for epigenetic dysregulation. The approach works because normal cells don’t depend on this aberrant metabolite—a selectivity advantage over traditional chemotherapy.
Serine starvation therapies take a different approach. Compounds targeting phosphoglycerate dehydrogenase (PHGDH), the rate-limiting enzyme in serine synthesis, selectively kill tumors that have lost the ability to import extracellular serine. Some designs incorporate prodrug elements activated only in low-serine environments—creating built-in selectivity for tumor microenvironments.
The most innovative approaches target metabolite transporters rather than enzymes. Inhibitors of the cystine-glutamate antiporter xCT starve tumors of reduced glutathione while sparing normal cells that can obtain cysteine through alternative pathways. This exploits cancer cells’ frequent loss of complementary nutrient import mechanisms.
Emerging work combines these strategies with metabolic imaging. PET tracers tracking tumor uptake of specific metabolites help identify which cancers will respond to which metabolic interventions—a form of precision nutrition warfare against malignancies.
Kinase Covalent Inhibition: Silencing Rogue Signaling at the Source
The rise of covalent kinase inhibitors represents a paradigm shift in targeting hyperactive enzymes that drive disease. Unlike traditional competitive inhibitors that must outcompete ATP, these drugs form permanent or long-lasting bonds with specific cysteine or lysine residues in their target kinases—a strategy particularly effective against resistance-prone mutants.
EGFR’s C797 residue exemplifies an ideal covalent target. Osimertinib’s acrylamide group forms a Michael addition with this nucleophilic cysteine, irreversibly blocking the kinase domain in lung cancer cells. Structural biologists note that the drug’s specificity stems from its requirement for both precise spatial alignment with C797 and the unique electrostatic environment around EGFR’s ATP-binding pocket.
BTK inhibitors like ibrutinib demonstrate another advantage—covalent binding allows sustained target suppression despite short plasma half-lives. The drug’s slow off-rate from Bruton’s tyrosine kinase means intermittent dosing can maintain effective inhibition, reducing toxicity compared to continuous high-dose regimens.
The field is moving beyond cysteine targeting. New warheads like sulfonyl fluorides can react with tyrosine or lysine residues, expanding the druggable kinome. Some designs incorporate light-activated protecting groups that only reveal reactive moieties at tumor sites—sparing normal tissues from off-target covalent modification.
Resistance remains a challenge, but next-generation covalent inhibitors are fighting back. Compounds like BLU-945 target both canonical resistance mutations and the wild-type EGFR allele simultaneously, while others exploit allosteric cysteines that emerge as escape mutations—turning resistance mechanisms into new drug targets.
Epigenetic Enzyme Trapping: Locking Down Dysregulated Writers and Erasers
Aberrant epigenetic modifying enzymes—the “writers” and “erasers” of DNA and histone marks—are being targeted by drugs that trap them in nonproductive complexes rather than simply inhibiting their catalytic activity. This approach has proven particularly effective against chromatin regulators that operate through multimeric complexes and DNA recognition.
EZH2 inhibitors exemplify this strategy. Tazemetostat doesn’t just block this histone methyltransferase’s SET domain—it stabilizes the enzyme in a conformation that prevents proper engagement with nucleosome substrates. Structural biologists emphasize that this “complex trapping” provides more sustained suppression of H3K27 methylation than catalytic inhibition alone.
BET bromodomain inhibitors work similarly. Compounds like JQ1 don’t merely compete with acetyl-lysine binding—they induce conformational changes that prevent BET proteins from recruiting their transcriptional machinery partners. This explains their efficacy against cancers dependent on specific enhancer programs.
The most sophisticated approaches target protein-protein interfaces. Some experimental drugs bind the scaffolding regions of epigenetic complexes rather than catalytic sites, disrupting higher-order organization essential for function. Others exploit allosteric pockets that only emerge when these enzymes engage their native substrates.
Emerging work focuses on temporal control. Photocaged epigenetic inhibitors allow light-directed activation at specific genomic loci—a precision approach that could minimize the widespread transcriptional effects seen with systemic administration.
Metabolic Synthetic Lethality: Killing Cells by Dual Pathway Blockade
The principle of synthetic lethality—where only simultaneous inhibition of two pathways is fatal—has found fertile ground in targeting metabolic redundancies. Cancer cells frequently delete backup metabolic genes to conserve resources, creating vulnerabilities invisible to single-agent approaches.
PARP inhibitors’ success against BRCA-deficient tumors inspired broader applications. Many tumors disable DNA repair pathways like mismatch repair or translesion synthesis while becoming dependent on parallel metabolic routes like nucleotide salvage pathways—dependencies now being exploited by selective inhibitors.
MTHFD2 inhibitors represent one promising class. This mitochondrial folate enzyme becomes essential in many cancers that downregulate cytosolic isoforms. When combined with inhibitors of alternative one-carbon pathways, MTHFD2 blockade proves selectively toxic to tumors.
The approach extends beyond cancer. Infectious disease researchers are identifying pathogen-specific metabolic redundancies—like malaria parasites’ dual dependence on glycolysis and gluconeogenesis—that can be simultaneously targeted for selective antimicrobial effects.
The challenge lies in patient stratification. Advanced metabolomic profiling now identifies tumors with specific pathway deletions that predict synthetic lethal responses—a precision medicine approach that could maximize efficacy while minimizing toxicity.
Allosteric Hijacking: Remote Control of Dysregulated Enzymes
Allosteric inhibitors that bind outside enzyme active sites are providing unprecedented control over pathological proteins resistant to conventional inhibition. These compounds exploit evolutionary control points that regulate enzyme activity naturally—often with better specificity than active-site competitors.
mTOR’s rapamycin-binding domain exemplifies an ideal allosteric target. Everolimus doesn’t block mTOR’s kinase domain directly—it stabilizes an inhibitory interaction with FKBP12 that prevents substrate access. This indirect mechanism allows selective inhibition of some mTOR functions while sparing others.
The field is advancing beyond natural allosteric sites. Fragment-based screening and cryo-EM have revealed cryptic pockets that emerge during enzyme dynamics—like the “switch-control” pocket in KRAS that AMG 510 exploits to lock the oncoprotein in an inactive state.
Some designs incorporate allosteric cooperativity. Bivalent molecules that simultaneously bind active and allosteric sites can achieve super-stoichiometric inhibition—a single inhibitor molecule silencing multiple enzyme copies through induced clustering.
The frontier involves light-sensitive allosteric modulators. Photoswitchable compounds that alter conformation under specific wavelengths allow spatiotemporal control over enzyme activity—potentially enabling tissue-specific metabolic modulation without systemic effects.
Proteolysis-Targeting Chimeras (PROTACs): Forcing Rogue Enzymes into Oblivion
PROTACs represent a radical departure from conventional inhibition—these heterobifunctional molecules don’t merely block pathological enzymes but tag them for complete destruction by the cell’s ubiquitin-proteasome system. This approach is particularly effective against targets that resist traditional inhibition.
ARV-471 illustrates the advantages. This estrogen receptor degrader eliminates both the ligand-binding domain and constitutively active splice variants that selective estrogen receptor modulators can’t address—overcoming a major resistance mechanism in breast cancer.
The technology excels against scaffold proteins. Traditional inhibitors struggle against non-enzymatic proteins like transcription factors or structural elements, but PROTACs can target these for degradation by hijacking E3 ligase recognition.
The field is overcoming early limitations. New E3 ligase recruiters with tissue-specific expression patterns are reducing off-target effects, while linker optimizations improve pharmacokinetics. Some designs incorporate disease-environment triggers that only activate degradation in pathological contexts.
Emerging work focuses on expanding the degradation toolkit. Autophagy-targeting chimeras (AUTACs) and lysosome-targeting chimeras (LYTACs) are being developed for targets the proteasome can’t handle—greatly expanding the druggable proteome.
Metabolic Checkpoint Immunotherapy: Rewiring Immune Cell Biochemistry
The latest frontier in immunotherapy involves small molecules that modulate immune cell metabolism to overcome suppression in the tumor microenvironment. Unlike biologics that target immune checkpoints directly, these drugs alter the biochemical infrastructure of immune evasion.
IDO1/TDO2 inhibitors exemplify this approach. By blocking tryptophan catabolism to kynurenine, these compounds reverse the immunosuppressive metabolic reprogramming induced by tumors—effectively removing the biochemical “brakes” on T cell function.
The field is moving beyond simple enzyme inhibition. Some compounds mimic the metabolic state of activated T cells—like PEP analogs that enhance antitumor activity by bypassing glycolytic blocks. Others provide metabolic support—such as glutamine analogs that selectively rescue exhausted T cells from tumor-induced starvation.
The most innovative approaches involve dual immunometabolic modulation. Compounds that simultaneously inhibit tumor lactate export while boosting T cell lactate import create a metabolic “sink” that selectively starves immunosuppressive cells in the microenvironment.
Future directions include temporal control. Pulsing immunometabolic drugs in rhythms that match T cell activation cycles may maximize efficacy while minimizing toxicity—a chronotherapeutic approach to immune reprogramming.
Enzyme Reactivation: Rescuing Pathologically Silenced Proteins
While most drug discovery focuses on inhibiting rogue enzymes, a growing class aims to resuscitate pathologically silenced ones—particularly for neurodegenerative and genetic metabolic disorders. These compounds operate through mechanisms far more sophisticated than simple agonist binding.
GCase chaperones for Gaucher’s disease illustrate the approach. Ambroxol and similar compounds don’t just boost residual enzyme activity—they stabilize misfolded glucocerebrosidase variants in functional conformations long enough to reach lysosomes.
The field is advancing beyond molecular crutches. Some compounds bind allosteric sites to increase mutant enzymes’ affinity for natural substrates. Others promote proper trafficking by mimicking endoplasmic reticulum quality control signals that mutant proteins fail to recognize.
The most sophisticated designs address multiple defects simultaneously. Bifunctional molecules might stabilize a misfolded enzyme’s tertiary structure while also enhancing its binding to lysosomal transport proteins—a coordinated rescue that single-mechanism drugs can’t achieve.
Emerging work focuses on reactivation specificity. Tissue-targeted enzyme chaperones and conditionally active stabilizers are in development to avoid off-target effects while maximizing delivery to affected organs.
The New Grammar of Pharmacological Intervention
The drugs emerging from metabolic and enzymatic precision targeting represent more than just new compounds—they embody a fundamental evolution in how we conceptualize pharmacological intervention. Where traditional drugs sought to block or activate, these agents reprogram, redirect, and sometimes entirely eliminate pathological biochemical entities.
This shift from inhibition to precise metabolic manipulation opens therapeutic possibilities for diseases long considered undruggable. From rescuing silenced enzymes to starving tumors of their idiosyncratic metabolic needs, the strategies are as diverse as the biochemical pathologies they address.
The future lies in combining these approaches—perhaps a PROTAC that degrades a rogue kinase while a metabolic inhibitor starves its downstream effects, or an allosteric activator that rescues a deficient enzyme while a covalent inhibitor silences its pathological competitor.
In this new era of drug design, molecules don’t merely interact with biology—they rewrite its faulty code with the precision of master programmers. The grammar of pharmacology will never be the same.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts
Medicinal Chemistry & Pharmacology
Polarity Alchemy: Strategic Charge Manipulation in Contemporary Drug Design
The future promises tunable therapies with polarity adjustable by light, magnetic fields, or bioorthogonal triggers.
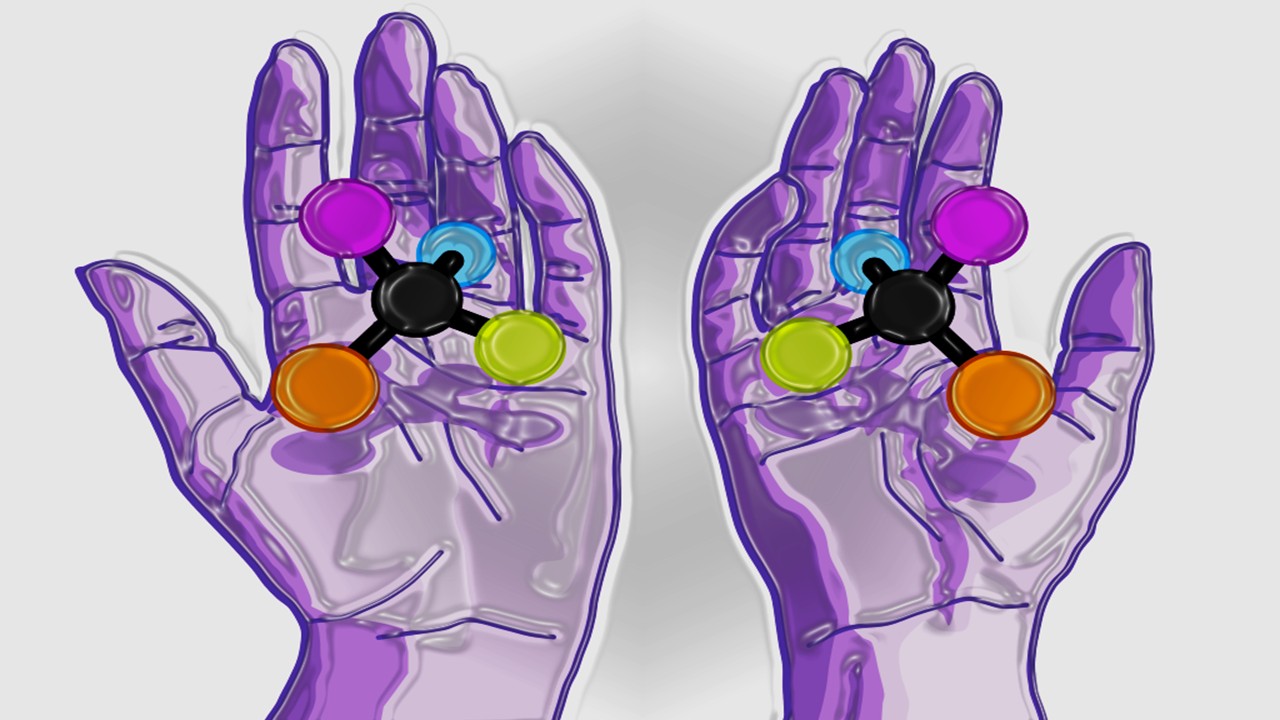
Medicinal Chemistry & Pharmacology
Chiral Warfare: How Mirror Molecules Are Revolutionizing Drug Design
Mirror-image molecules, known as enantiomers, are no longer mere curiosities but powerful tools reshaping drug discovery.
Read More Articles
Chemical Gale: How Wind Energy is Reshaping Industrial Manufacturing
The integration of wind energy into chemical manufacturing constitutes a fundamental reimagining of process chemistry.
Algorithmic Trials: How Decision Theory is Reshaping Decentralized Clinical Research
Decision theory offers a robust mathematical framework to design trials that enhance efficiency, uphold ethical standards, and better reflect the complexities of real-world therapeutic contexts.