In the quest for better medicines, the philosopher Peter Singer’s words resonate: improvement is the name of the game. One area ripe for evolution is preclinical drug development, traditionally reliant on animal testing. However, innovative alternatives are emerging, promising faster, cheaper, and potentially more accurate insights into drug efficacy and safety.
In Vitro and In Silico
The traditional reliance on animal testing faces formidable challenges, prompting a shift towards in vitro and in silico methods. In vitro approaches, leveraging cultured cells, offer speed and cost advantages but grapple with issues such as genetic instability and non-physiological conditions. However, advancements like Good Cell Culture Practice are mitigating these concerns.
Concurrently, in silico methods, powered by artificial intelligence, are revolutionizing preclinical testing landscapes. Tools like Good Read-Across Practices and automated read-across harness vast toxicological databases, enhancing drug discovery endeavors. Integrated testing strategies (ITS) are emerging, marrying in vitro and in silico methods to provide comprehensive data, recognizing the limitations of individual approaches.
Mechanistic Insights
The paradigm shift towards mechanistic research in biochemistry and molecular biology is illuminating disease pathways with unprecedented clarity. Unlike traditional animal models, mechanistic studies delve into cellular and molecular aspects, paving the way for surrogate measures (“biomarkers”) that streamline clinical trials and enable early efficacy assessments—a cornerstone of translational medicine.
Bridging the Gap between Animal Models and Human Responses
Microphysiological systems (MPS), epitomized by organs on chips, offer a tantalizing glimpse into the future of drug testing. These innovative platforms replicate human organ functionalities with unprecedented fidelity, leveraging microfluidic technologies to mimic blood flow and tissue interactions. MPS not only model complex diseases but also enable personalized medicine by testing patient-derived cells.
Enhancing Drug Safety Paradigms
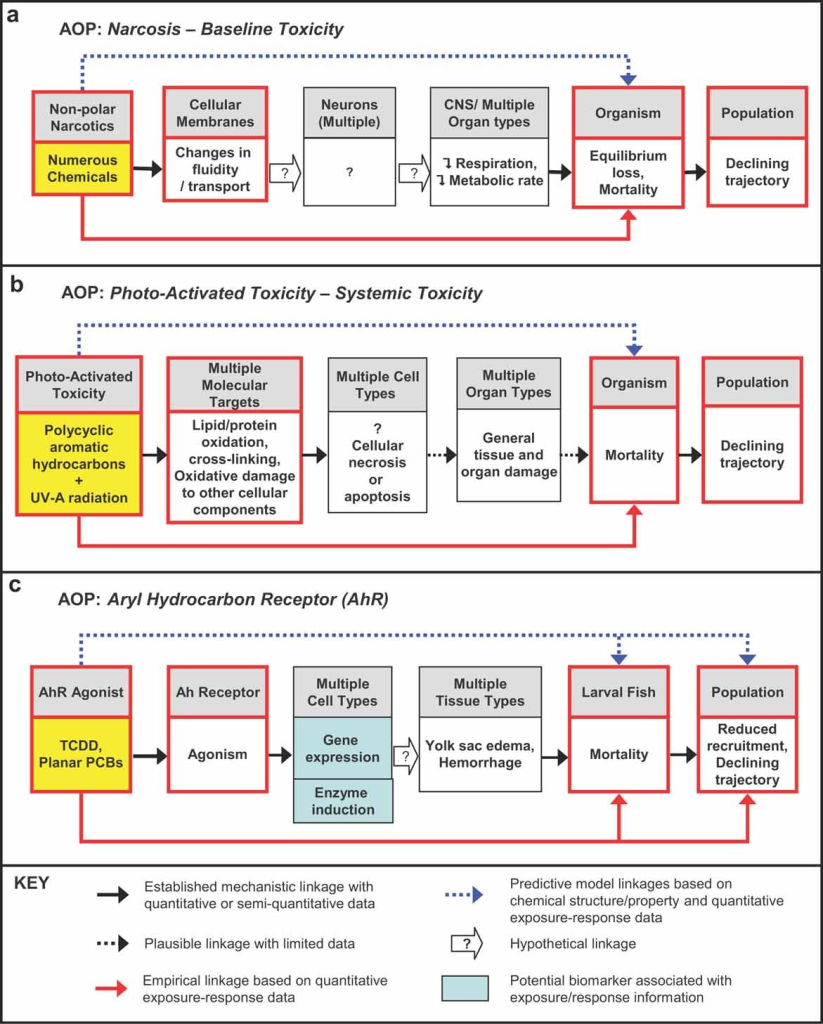
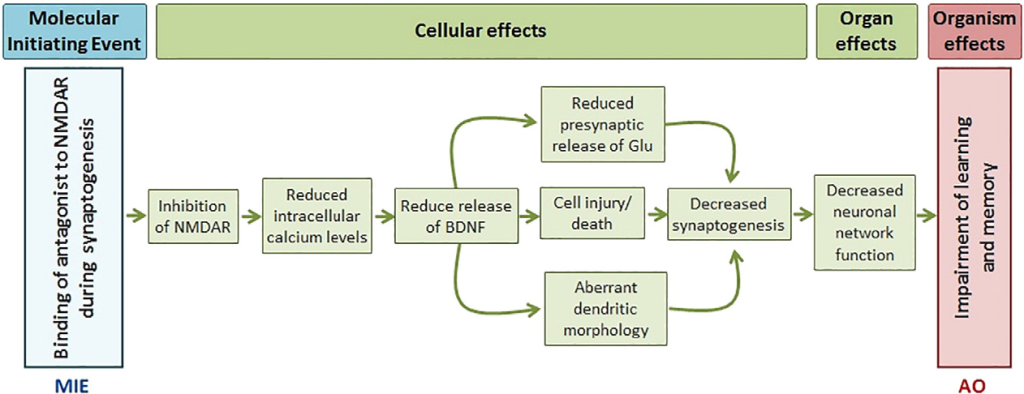
The advent of adverse outcome pathways (AOPs) heralds a new era in drug safety assessment. By delineating the cascade of events from chemical exposure to adverse health effects, AOPs provide mechanistic insights crucial for early hazard identification. Combined with a pathway of toxicity approaches, AOPs offer a holistic understanding of toxicity mechanisms, steering drug development towards safer outcomes.
Harnessing the Power of Artificial Intelligence
Artificial intelligence (AI) stands at the vanguard of drug development, leveraging big data to predict toxicity, design drugs, and streamline risk assessment. From quantitative structure-activity relationships to deep learning models, AI transcends traditional boundaries, offering probabilistic risk modeling and mechanistic insights. However, challenges like biases and interpretability underscore the need for multidisciplinary collaboration to harness AI’s full potential responsibly.
Redefining Toxicity Testing
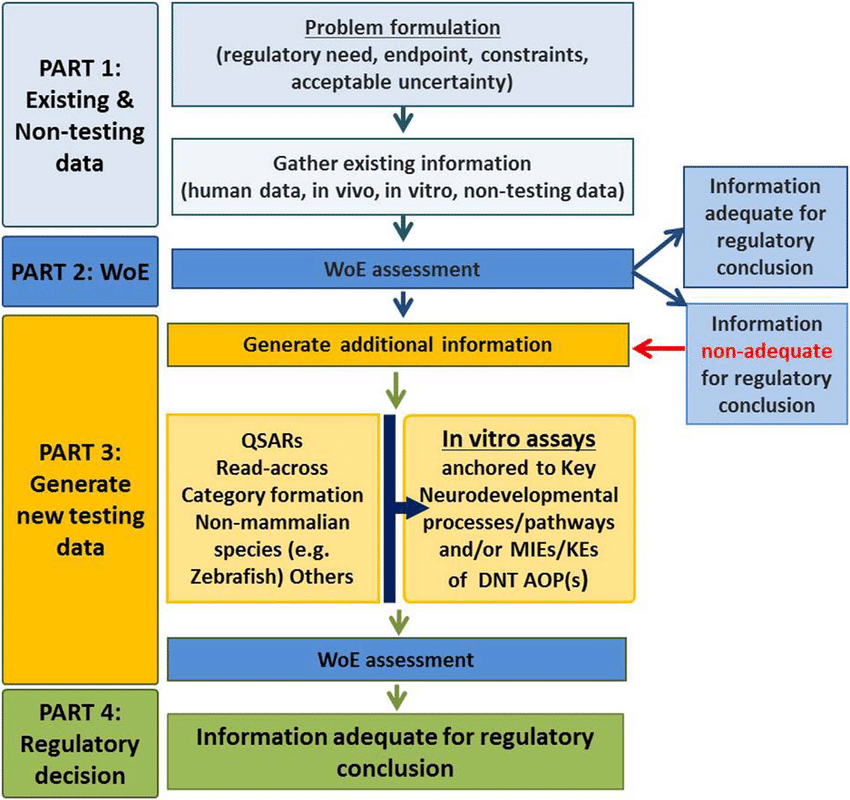
Integrated approaches to testing and assessment (IATA) epitomize a paradigm shift towards human-relevant toxicology. By synergizing diverse testing methodologies, IATAs minimize reliance on animal models while maximizing predictive accuracy. Challenges like regulatory acceptance and test validation persist, but IATAs represent a pragmatic step towards a more ethical and efficient drug development landscape.
Embracing Animal Research: Navigating the Paradox
Despite strides towards alternative methodologies, animal research remains indispensable in drug development. From exploring disease mechanisms to setting initial safety parameters, animals play a pivotal role in bridging the gap between bench and bedside. Strategies to maximize the value of animal data, coupled with ongoing advancements in alternative testing, underscore the nuanced approach necessary for sustainable progress in drug development.
In Pursuit of Progress: The Road Ahead
The pursuit of better medicines is a journey fraught with challenges and opportunities. As we navigate the complexities of drug development, a harmonious blend of traditional wisdom and innovative technologies will be paramount. The future of medicine beckons — a future where every patient’s journey is guided by precision, efficacy, and compassion.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts
Drug Discovery Biology
Breaking Barriers: The Science Behind Designing Membrane-Disrupting Therapeutics
Membrane-disrupting agents offer a powerful therapeutic strategy by collapsing diseased cell membranes with surgical precision and structural specificity.
Drug Discovery Biology
Mini Organs, Major Breakthroughs: How Chemical Innovation and Organoids Are Transforming Drug Discovery
By merging chemical innovation with liver organoids and microfluidics, researchers are transforming drug discovery into a biologically precise, patient-informed, and toxicity-aware process.
Read More Articles
Tetravalent Vaccines: The Power of Multivalent E Dimers on Liposomes to Eliminate Immune Interference in Dengue
For the first time, a dengue vaccine candidate has demonstrated the elusive trifecta of broad coverage, balanced immunity, and minimal enhancement risk,
Primed by Parasites: How Malaria Exposure and Off-Target Immunity Shape RTS,S Vaccine Protection
RTS,S/AS02A leverages malaria’s immunological imprint to elicit both targeted and unexpected antibody responses, reshaping how we understand—and engineer—vaccine efficacy in exposed populations.