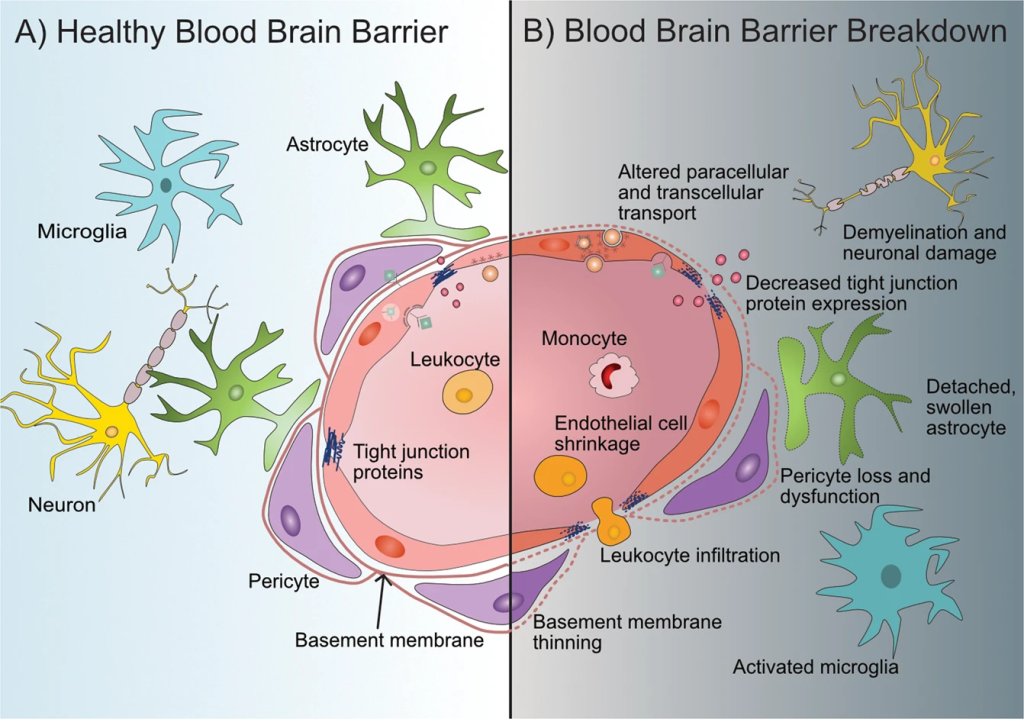
The limitations posed by the blood-brain barrier (BBB) and the drawbacks of current therapies underscore the urgent need for innovative approaches to treat neurodegenerative disorders (NDs). Among the various strategies being explored, nanotechnology stands out as a safe and promising platform for targeted drug and gene delivery to the central nervous system (CNS). This approach leverages materials at the nanoscale, typically ranging from 1 to 1000 nanometers, capable of interacting with biological systems at the molecular level.
The Promise of Nanocarriers
Nanocarriers, due to their unique properties, have proven to be highly effective in delivering drugs and genes to the brain. These properties include high drug loading capacity, low systemic toxicity, improved drug permeabilization, and robust physical and chemical stability. Various materials such as natural polymers (proteins and polysaccharides), synthetic polymers (PLGA and PCL), and inorganic substances (gold, silver, and cerium) are employed to formulate nanoparticles.
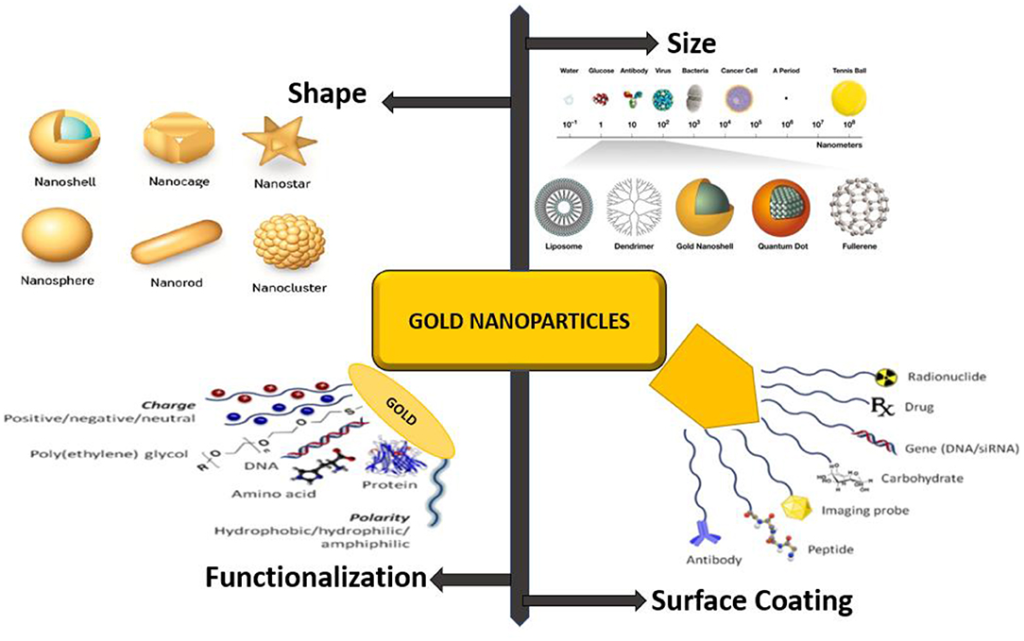
The ability of these nanoparticles to penetrate the BBB is influenced by factors such as size, surface chemistry, type, and polarity of the nanocarriers. Surface coatings with substances like polysorbate can further enhance their capability to bypass transmembrane efflux systems, making liposomal and polymeric nanoparticles particularly attractive for brain-targeted delivery. These nanoparticles can be easily modified with ligands and cell-penetrating peptides (CPPs), enhancing their specificity and efficiency.
Metal Nanoparticles: Pioneers in Brain Targeting
Gold Nanoparticles (AuNPs). These nanoparticles are extensively used in CNS imaging and targeting due to their plasmonic properties, which allow them to interact with electromagnetic radiation. This makes them ideal for imaging applications using micro-CT scanning or X-rays, providing higher contrast and precise visualization compared to conventional contrast agents. Recent studies have shown that gold nanoparticles, when complexed with compounds like rhodamine B isothiocyanate (RITC) and poly-L-lysine (PLL), significantly increase uptake in human mesenchymal stem cells (hMSC), enabling direct visualization post-injection into rat brains.
Furthermore, AuNPs have demonstrated potential in targeting and degrading β-amyloid aggregates, a hallmark of Alzheimer’s disease. By conjugating AuNPs with apolipoprotein E3 (ApoE3) and tracking with curcumin, researchers have observed significant dissociation of amyloid aggregates. Additionally, surface-modified AuNPs with brain-targeted exosomes have shown enhanced brain delivery, underscoring their adaptability and biocompatibility for delivering therapeutic molecules.
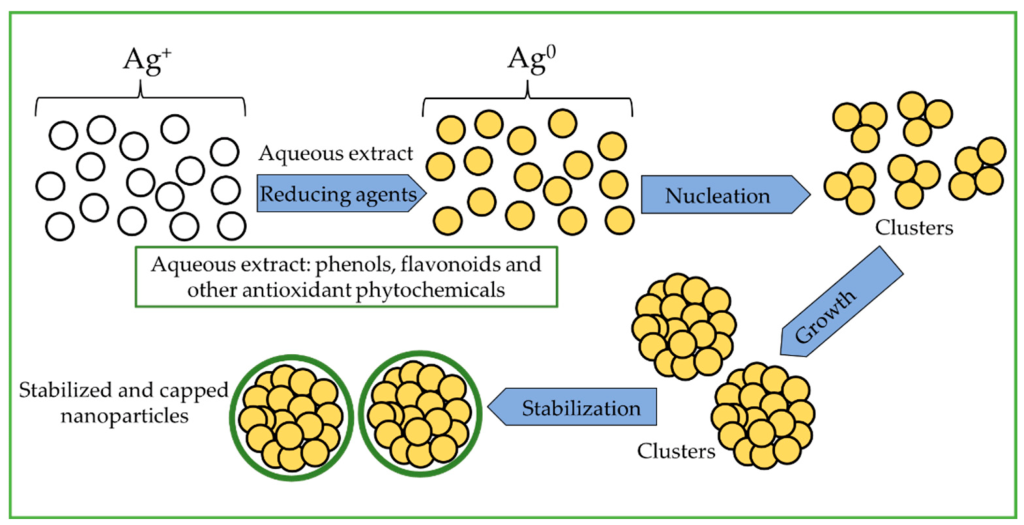
Silver Nanoparticles (AgNPs). AgNPs have been explored for their ability to accumulate in the hippocampus, a critical region for NDs. They have been used to deliver various drugs to the brain, including treatments for glioblastoma and brain-eating amoebae. Notably, AgNPs exhibit anti-inflammatory and antioxidant properties when absorbed by microglia, the brain’s immune cells. However, their entry into the brain involves disrupting the BBB, which can induce neuronal degeneration and necrosis due to long-term silver accumulation.
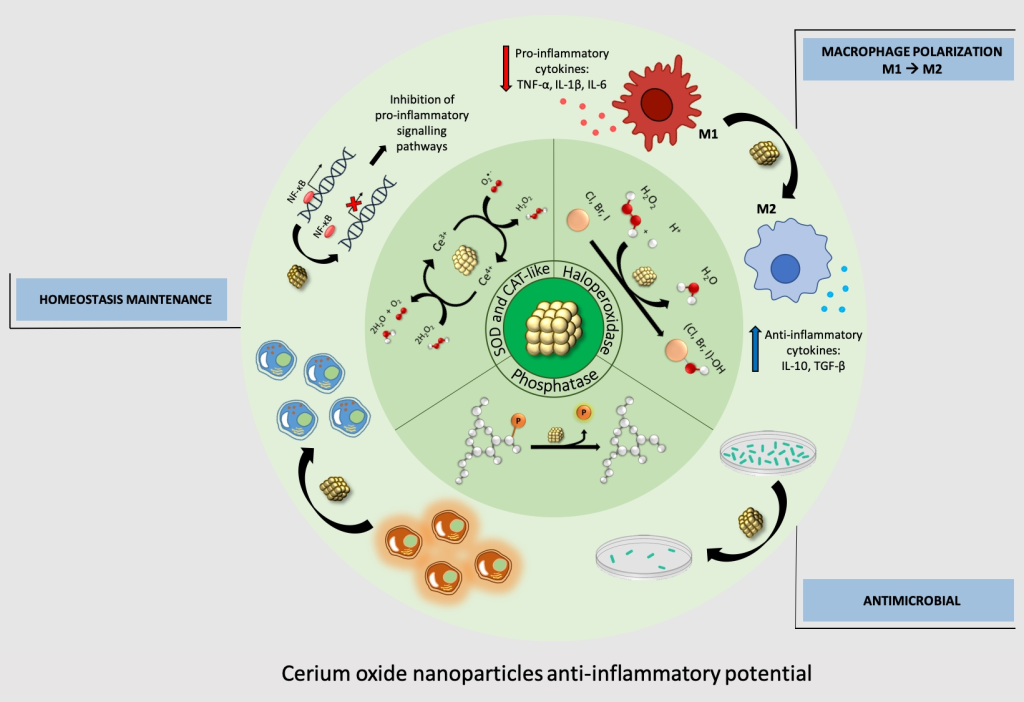
Cerium Oxide Nanoparticles. Known for their role in reducing reactive oxygen species (ROS), cerium oxide nanoparticles, also known as nanoceria, exhibit excellent antioxidant properties due to the transition between Ce+3 and Ce+4 oxidation states. They have shown potential in retarding apoptotic effects in Alzheimer’s disease, scavenging ROS in ischemic stroke models, and restoring motor function in multiple sclerosis and ALS models. Studies have also highlighted their neurogenesis efficacy, promoting neuronal regeneration and reducing inflammation in the hippocampal region.
Organic Nanoparticles: Biocompatibility and Efficiency
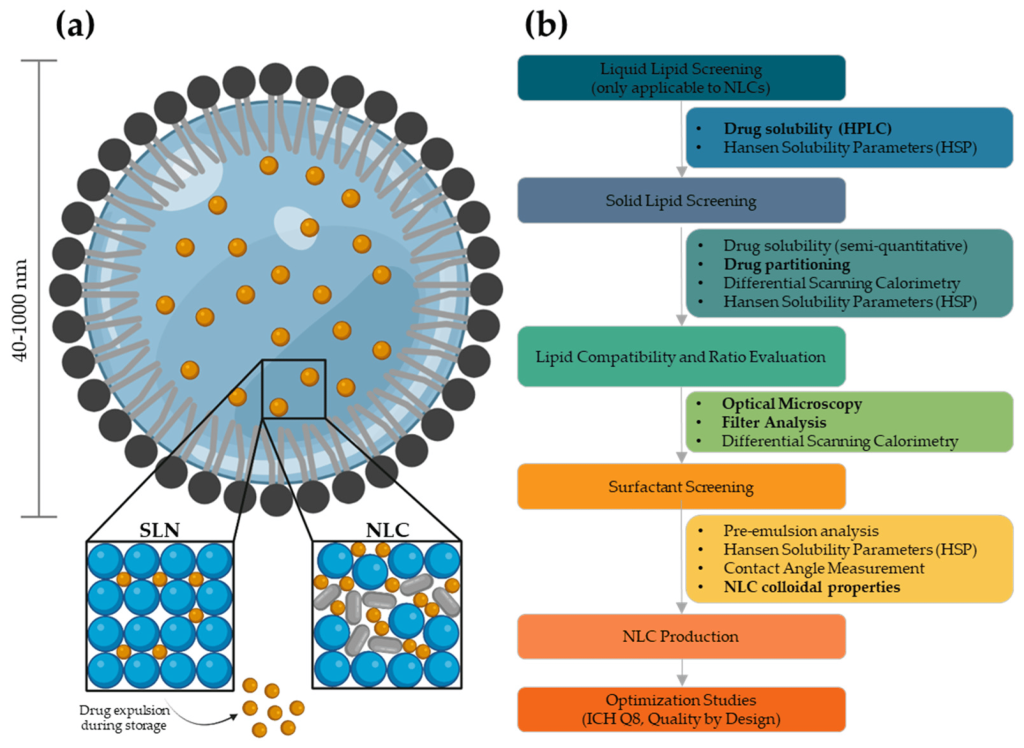
Lipid Nanocarriers. Among organic nanocarriers, liposomes have been extensively studied for brain-targeted delivery due to their biocompatibility and ability to protect therapeutic agents from degradation. Functionalized liposomes, dual-modified with targeting ligands like mApoE and cell-penetrating peptides, have shown enhanced BBB crossing and targeting of amyloid aggregates. These formulations have demonstrated the ability to disaggregate amyloid fibrils and improve drug accumulation in the brain, making them promising candidates for treating NDs.
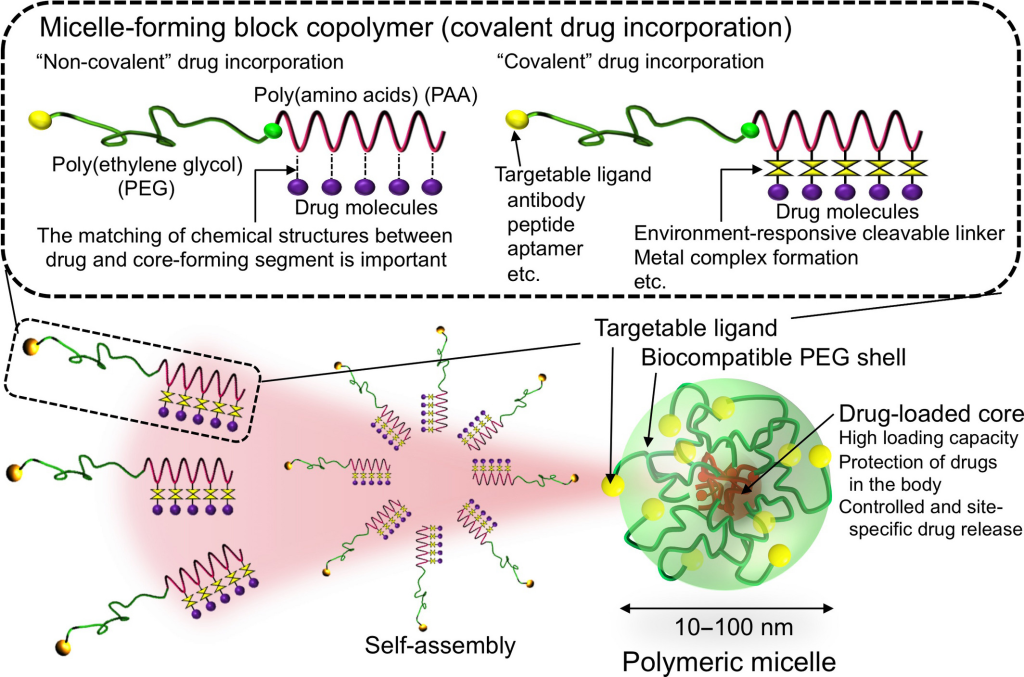
Polymeric Nanomicelles. These structures, formed from self-assembling polymers, have emerged as effective vehicles for delivering diverse therapeutic agents. Chitosan-based nanomicelles, in particular, offer advantages such as biodegradability, nontoxicity, and surface modification flexibility. Studies have shown their efficacy in transfecting brain cells and inhibiting α-syn aggregation, highlighting their potential for treating Parkinson’s disease.
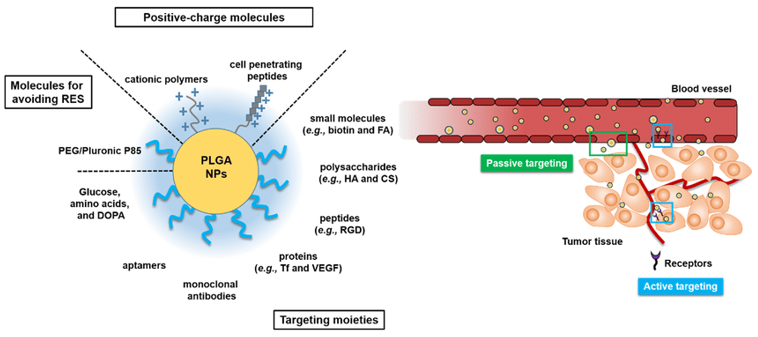
Poly D, L-(lactic-co-glycolic) Acid (PLGA). This biodegradable polymer is widely used for brain-targeted drug delivery due to its high drug loading capacity and ability to cross the BBB. PLGA nanoparticles, coated with peptides like TET1, have demonstrated successful delivery of hydrophilic drugs to the brain, reducing amyloid aggregation and showing potential as a treatment for Alzheimer’s disease.
Clinical Trials and Future Prospects
The potential of nanomedicine in treating NDs is evident from ongoing clinical trials. Although only a few nanoparticle-based formulations are currently in trials, the results are promising. For instance, lipid nanoparticle formulations for transthyretin-mediated amyloidosis and CRISPR/Cas9 gene-based studies are advancing through various trial phases. The development of gold nanoparticle-mediated delivery systems for ALS and other NDs is also progressing.
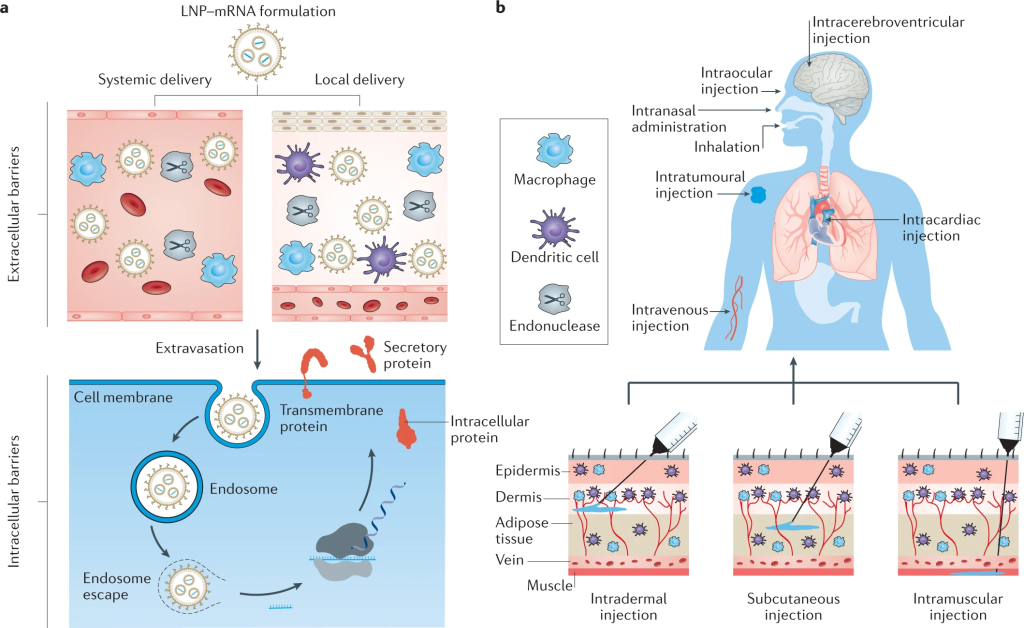
Overcoming Challenges
Despite the promising advances, challenges remain in nanoparticle-mediated drug delivery to the brain. Factors such as nanoparticle toxicity, production consistency, and regulatory approval need to be addressed. Toxicity depends on size, surface charge, and shape, requiring careful consideration and surface modification to minimize adverse effects. Uniform production methods and thorough pharmacokinetic studies are essential for ensuring efficacy and safety. Regulatory frameworks must evolve to accommodate the unique aspects of nanotherapeutics.
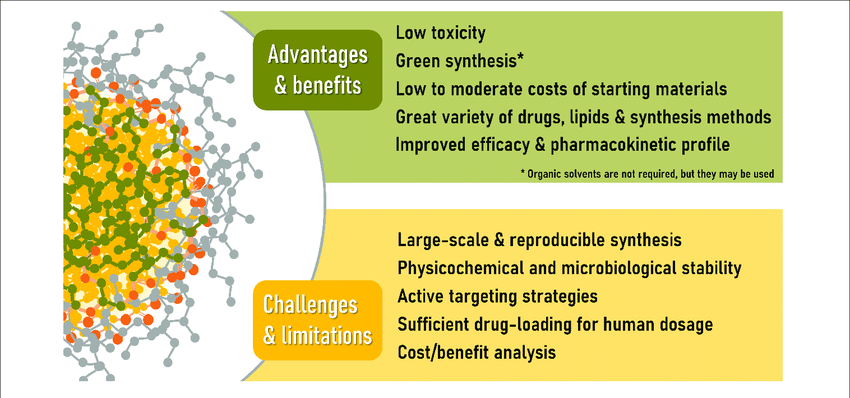
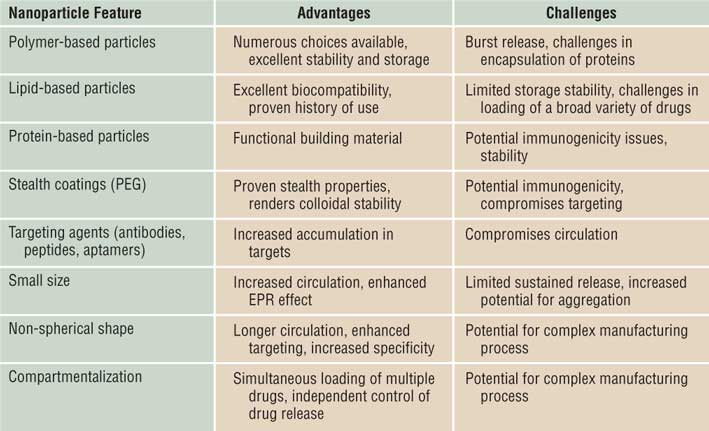
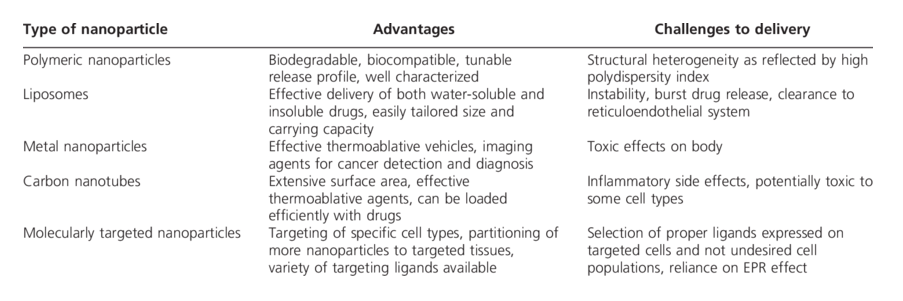
Nanomedicine offers a hopeful avenue for treating NDs, with the potential to overcome the limitations of traditional therapies. Continued research, in vitro and in vivo studies, and clinical trials are crucial for translating these promising findings into effective treatments. The future of nanomedicine holds the promise of halting neurodegeneration and improving the lives of those affected by these debilitating disorders.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.

Medicinal Chemistry & Pharmacology
Coprocessed for Compression: Reengineering Metformin Hydrochloride with Hydroxypropyl Cellulose via Coprecipitation for Direct Compression Enhancement
In manufacturing, minimizing granulation lines, drying tunnels, and multiple milling stages reduces equipment costs, process footprint, and energy consumption.

Medicinal Chemistry & Pharmacology
Decoding Molecular Libraries: Error-Resilient Sequencing Analysis and Multidimensional Pattern Recognition
tagFinder exemplifies the convergence of computational innovation and chemical biology, offering a robust framework to navigate the complexities of DNA-encoded science
Read More Articles
Magnetic Nanoengineering: Overcoming Biological Variability and Enhancing Therapeutic Precision
The future of nanomedicine lies in harmonizing precision, accessibility, and ecological responsibility, ushering in an era where therapies are tailored to individual biological landscapes.