Drug v. Poison: Which is Which?
The difference between a drug and a poison is the dose. This is a common quotation attributed to Paracelsus.
Swiss physician, alchemist, lay theologian, and philosopher Paracelsus, also known by his very long name Philippus Aureolus Theophrastus Bombastus von Hohenheim, lived during the German Renaissance. He was a pioneer in various areas of the “medical revolution” of the Renaissance, emphasizing the value of observation in combination with accepted wisdom.
In his Third Defence, he explained the idea of dosage response in great detail, saying that “Solely the dose determines that a thing is not a poison” (Sola dosis facit venenum “Only the dose makes the poison”). Outsiders regularly attacked Paracelsus’s use of inorganic materials in medicine, claiming that his use of chemical agents was too hazardous for medicinal purposes. His concept that illnesses are located in a particular organ was expanded to include target organ toxicity, which states that there is a particular location in the body where a chemical will have the most impact.

Additionally, Paracelsus advocated utilizing laboratory animals to investigate both advantageous and harmful pharmacological effects. One of the earliest scientists to apply chemistry to medicine was Paracelsus.
He is, thus, known as the “father of toxicology” because of his vast experiments.
Antidotes: Life-Saving Yet Still of Limited Scope
While in some respects the tenets religiously held and belligerently defended by Paracelsus are found to be correct, and we are, of course, all grateful to him for springboarding the study of doses and poisons, the simplistic fluid imbalance theory that he so devotedly presented as fact indubitably needed for modern medicine and contemporary pharmacy to take over.
Forward to the 21st century, we have here the following table of antidotes obtained from the 58th Chapter (Management of the Poisoned Patient) of the 9th Section (Toxicology) of the 14th Edition (2018) of Katzung’s Basic and Clinical Pharmacology. Bertram G. Katzung, MD, PhD, is a Professor Emeritus of the Department of Cellular and Molecular Pharmacology of the University of California, San Francisco.
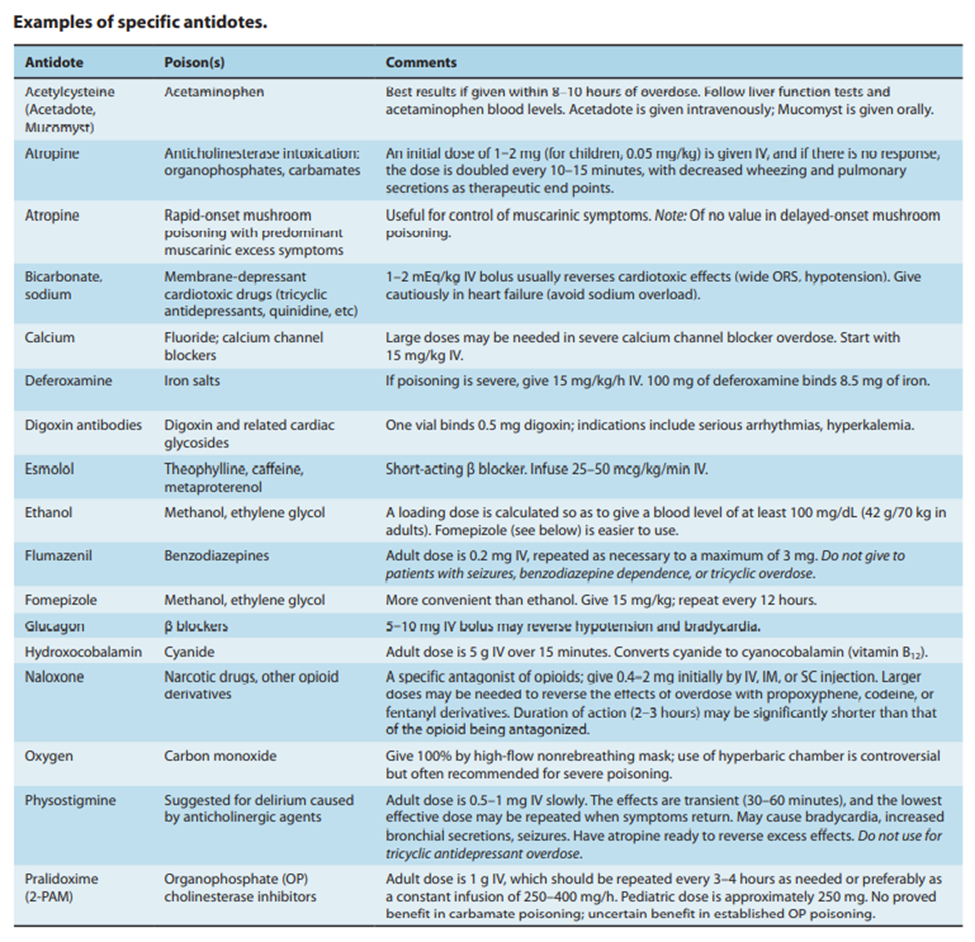
From this table, we can determine that opioid overdoses, such as with fentanyl and heroin, are currently treated with naloxone.
Naloxone antagonistically acts on mu opioid receptors resulting to presynaptic inhibition as an expected outcome of reduced Ca2+ conductance and decreased levels of the secondary messenger cyclic adenosine monophosphate (cAMP). Postsynaptically, an inhibition is as well exhibited by naloxone on delta and kappa opioid receptors via augmentation of K+ conductance and consistent reduction of cAMP levels.
Despite the conspicuous pharmacological effectivity of the opioid receptor antagonist naloxone, overdoses with non-opioid medications will render the drug product a useless antidote. This is where antibodies come into play.
Determined to have significant capacity for treatment of overdosage, antibodies show remarkable binding properties to certain drugs in the bloodstream as with the cases of cocaine and methamphetamine toxicities. In addition, the sequestration ability is rather further praised for its ability to prevent entry into the blood-brain barrier (BBB).
Nevertheless, it is an undeniable fact that the employment of antibodies proves not only a costly preparation, but also requires exacting storage conditions under well-regulated environments since they are prone to degradation.
Mission Sequestration with Supramolecular Chemistry
A potential broad-spectrum overdose treatment has been discovered by Lyle Isaacs and his colleagues at the University of Maryland in the US by utilizing the benefits of host-guest chemistry. The study of host-guest chemistry makes use of supramolecular structures as hosts for chemical reactivity. The chemicals discovered by Isaacs and his colleagues are made using a quick, inexpensive three-step synthetic organic chemistry procedure that is easily scalable to multigram scale. They are demonstrated to have excellent thermal, chemical, and aqueous solubility.
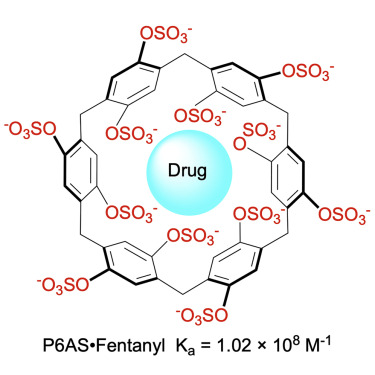
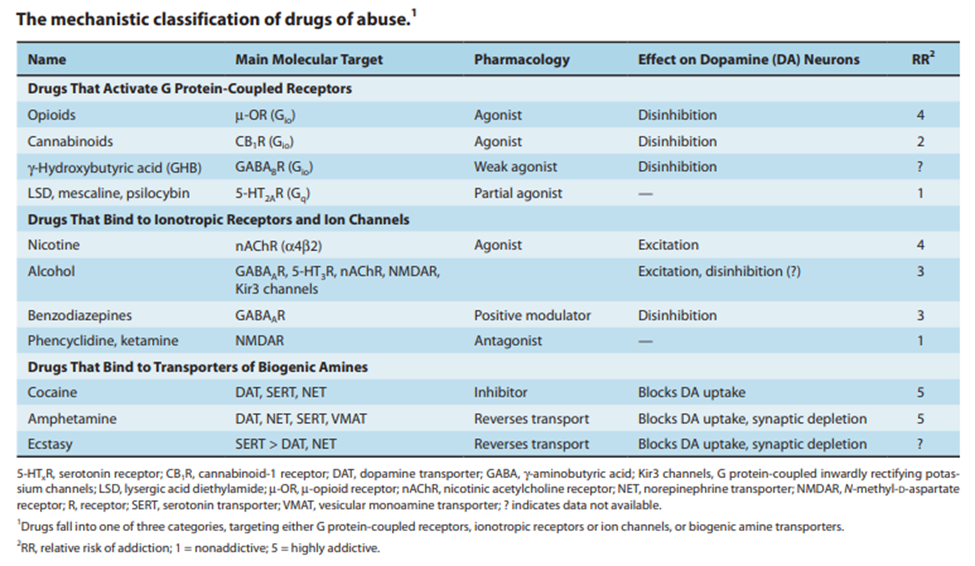
Among other drugs of abuse, such as opioids, stimulants, and hallucinogens, some of which currently lack a reversal agent, such as methamphetamine and phencyclidine (PCP), a macrocyclic molecule has demonstrated potential as a broad-spectrum antidote. When delivered after an overdose, the pillararene compound P6AS can sequester drug molecules inside of itself, altering their characteristics and lowering the likelihood of fatalities.
Because investigations in the previous ten years had revealed that pillarene compounds, macrocycles that form host-guest complexes, might counteract the effects of anesthetics and the insecticide paraquat, the researchers concentrated on these compounds. Additionally, Isaac’s team found in 2020 that sulfated pillararenes had a significant affinity for water’s hydrophobic cations. Since many drugs of abuse have hydrophobic cations, pillararenes may be able to remove them from the bloodstream.
The scientists tested the four pillararenes’ ability to bind to a variety of substances, including methamphetamine, fentanyl, MDMA (3,4-Methylenedioxymethamphetamine, or ecstasy), PCP, and mephedrone, in order to find out. The results confirmed that these medications all attach to pillarenes in water, with particularly significant binding to the pillarene P6AS. P6AS displayed great binding affinity to a broad spectrum of drugs of abuse, providing it promising value as a broad spectrum therapeutic for substance intoxication.
Benchmark for Future Soluble Sequestrants
The macrocycle P6AS has six p-phenylene “walls” with a total of 12 substituents that surround a hydrophobic cavity. This makes it possible for P6AS to bind to hydrophobic cations thanks to a combination of the hydrophobic effect, non-covalent π interactions between the aromatic rings of the drugs and the host’s walls, and electrostatic ion-ion interactions between the sulfate substituents and the cationic ammonium ion of the drug.
P6AS does not impede an ion channel that is essential for heart function or result in cell mutation, according to in vitro tests. The group also established the maximum acceptable dose of P6AS that could be administered to animals without causing harm. In experiments with mice given doses of methamphetamine or fentanyl, the narcotic effects were shown to be reversed when the mice were given intravenous injections of P6AS five to fifteen minutes later. Additionally, it worked just as well as naloxone as a fentanyl reversal agent.
The key to developing various classes of macrocycles is to find a practical use, particularly one that is related to therapeutic or consumer use, and this frequently calls for water-soluble derivatives. Werner Nau, a supramolecular chemist at Constructor University in Bremen, Germany, provided this significant observation. This standard is set by the current work for pillararenes, which builds on earlier, ground-breaking applications for other macrocycles. Another factor to consider is the strong affinity of sulfated pillararenes for methamphetamine and fentanyl.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Invisible Couriers: How Lab-on-Chip Technologies Are Rewriting the Future of Disease Diagnosis
The shift from benchtop Western blots to on-chip, real-time protein detection represents more than just technical progress—it is a shift in epistemology.

Medicinal Chemistry & Pharmacology
Designing Better Sugar Stoppers: Engineering Selective α-Glucosidase Inhibitors via Fragment-Based Dynamic Chemistry
One of the most pressing challenges in anti-diabetic therapy is reducing the unpleasant and often debilitating gastrointestinal side effects that accompany α-amylase inhibition.

Medicinal Chemistry & Pharmacology
Into the Genomic Unknown: The Hunt for Drug Targets in the Human Proteome’s Blind Spots
The proteomic darkness is not empty. It is rich with uncharacterized function, latent therapeutic potential, and untapped biological narratives.

Medicinal Chemistry & Pharmacology
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.