Quotient Sciences has established a solid track record for creating both straightforward and intricate fit-for-phase formulations for peptides and small compounds during the past 30 years. With its most recent investment, the business has increased the capacity of its high containment lab to support oral medicine applications, such as immediate release, modified release tablets, and multi-particulate dosage forms for highly powerful chemicals.
The manufacturing and drug development accelerator Quotient Sciences said today that it has finished the expansion of its Nottingham, UK facility’s early-phase formulation development capabilities for oral dosage forms. Via the company’s flagship platform, Translational Pharmaceutics®, the enhanced offerings increase the ability to assist with seamlessly integrated drug development initiatives by building on the site’s current formulation capabilities.
Quotient Sciences Formulation Development Services
Excellent and high-quality formulation development is underpinned by biopharmaceutics & clinical knowledge.
With small compounds and peptides, Quotient Sciences has more than 30 years of experience creating a variety of formulations for a variety of applications. Quotient Sciences have developed more than 1,500 compounds and have competence in both basic and sophisticated dosage formulations. Because of Quotient Sciences‘ exceptional track record in clinical research, the company is completely knowledgeable of the requirements for developing formulations at every level of the development process, from preclinical to late stage.
Using the physicochemical and biopharmaceutical characteristics of the client’s molecule, Quotient Sciences collaborates with its customers to create the most effective formulation, ranging from fit-for-purpose preclinical and first-in-human (FIH) dosage forms to optimized drug products for late-stage development and market.
Utilizing its cutting-edge facilities in the United States and the United Kingdom, Quotient Sciences provides a thorough and customized formulation development service, and it has a global staff of over 200 formulation and analytical experts.
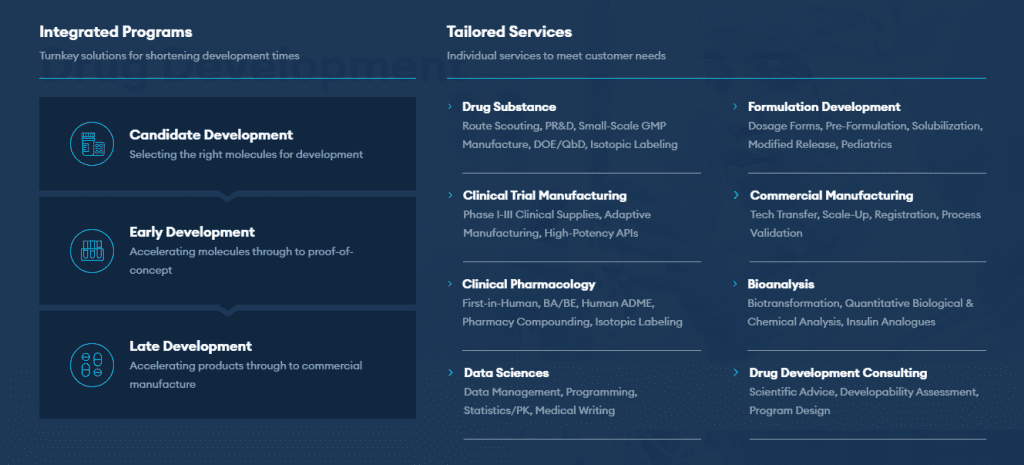
The following are the full range of Formulation Development Services offered by Quotient Sciences:
Access formulation design expertise in a variety of drug product formats at Quotient Sciences. The formulation teams at Quotient Sciences have a wealth of knowledge and a successful track record with a variety of medicinal product formats for oral, inhalation, topical, rectal, and parenteral delivery.
Access characterization techniques that speed up molecular development via Quotient Sciences‘ Pre-Formulation Screening services.
The skilled material scientists at Quotient Sciences can assist you with pre-formulation screening so that you can choose your drug candidate for preclinical testing and identify your drug product for early clinical testing.
Amorphous content, solubility screening, particle size analysis, and candidate stability are just a few of the methods used by Quotient Sciences‘ rapid screening approach to identify your lead candidates, which can then be developed into formulations for preclinical evaluation as either simple solutions/suspensions or more complex formulations.
Preclinical investigations can start sooner rather than later by quickly screening excipients and choosing the best formulations.
How does one select the most appropriate first-in-human formulation?
Quotient Sciences’ goal is on offering the most optimal formulations to speed your trial program, whether dosing within our own Phase I clinical facilities or at an external site. As necessary, Quotient Sciences works with you to enable quick progress to your first-in-human evaluation, making sure that your final goals for the introduction of your therapeutic product may be accomplished after confirmation of the safety and tolerability of the molecule. Years of experience fusing formulation development and clinical evaluation have prepared Quotient Sciences‘ formulators. Hence, a Quotient Sciences development product is based on knowledge of the target indication, including pharmaceuticals created for chronic dosage or for use in pediatric populations.
Overcome solubility challenges with your molecule with Quotient Sciences solubility enhancement services.
Given that insufficient water solubility affects over 70% of medications when they enter development, which makes it difficult for them to provide appropriate and reliable gastrointestinal absorption, Quotient Sciences provides a wide range of solutions to address your solubility issues. Quotient Sciences has developed a wide range of technologies and formulation strategies over the course of its nearly 30-year history to address these challenging solubility issues. The Quotient Sciences method has made it possible to significantly accelerate the optimization of your drug products for better oral bioavailability.
Optimize your modified-release formulations at Quotient Sciences.
There are several aspects related to patient and treatment need that influence the demand for modified-release formulations. Patients typically want once-daily or twice-daily treatments for the majority of illnesses, necessitating continuous medication concentrations throughout the course of treatment.
Modified-release formulations are made to administer your medication over a specified time period or to a specific area of the body. By balancing therapeutic need, managing AE profiles, and reducing dose frequency, it is possible to optimize drug administration and increase patient compliance.
From gastro-retention to sustained-release and pulsatile-release formats, Quotient Sciences has a wealth of experience in producing modified-release formulations.
How does one ensure a drug product’s performance?
Your drug research program’s success depends on having access to skilled analytical scientists and cutting-edge technology. The ability to deliver candidate screening, drug product analysis, and phase-appropriate method development and validation is one of Quotient Sciences‘ greatest strengths.
The material science specialists at Quotient Sciences have a track record of quickly identifying the characteristics of APIs to help the selection of lead drug product forms for pre-clinical and clinical testing.
Throughout the drug development process, Quotient Sciences is aware of your analytical goals and collaborates with you to make sure the right methodologies are accessible at every stage.
How are scintigraphic studies designed and conducted?
Gamma scintigraphy is a tried-and-true imaging method for observing how pharmacological dose forms operate in the body. With more than 25 years of experience in the design, execution, and reporting of scintigraphic investigations, Quotient Sciences is able to assist clients in comprehending how the composition of formulations, patient variables, and human physiology affect clinical outcomes.
To enable real-time monitoring of clinical performance after dosing in human patients, modest quantities of a gamma-emitting isotope are incorporated into the formulation during product production.
Drug administration via oral and inhalation routes is a key application.
Customize pediatric solutions at Quotient Sciences.
Patient needs and regulatory regulations have led to a critical aspect of the industry today: the creation of acceptable, edible pediatric formulations. Quotient Sciences can provide you a distinctive comprehensive pediatric development solution because of its vast expertise and skill set.
Scientists at Quotient Sciences have produced tailored pediatric pharmaceutical formulations that have been approved by the FDA and have extensive experience creating pleasant formulations for the OTC/consumer health care market.
Using a variety of taste-modifying and taste-masking approaches, the company has a long history of developing age-appropriate dosage forms of unpleasant, bitter medicinal compounds without sacrificing product stability and PK performance.
Prior to moving on to pivotal pediatric studies, Quotient Sciences conducts quick, adaptive trials in humans to optimize taste qualities and/or PK performance. This is done using the company’s integrated GMP manufacturing and clinical testing platform.
Corner Office Comments
According to Andy Lewis, Global Vice President of Integrated Pharmaceutical Sciences at Quotient Sciences, the company’s exceptional history in clinical research serves as the foundation for Quotient Sciences‘ expertise in formulation development. Quotient Sciences is able to speed up the development process for its clients because it has in-house expertise in both biopharmaceutics and clinical matters. This expertise, together with the company’s experience from creating more than 1,500 compounds, also helps.
With these increased capabilities, Quotient Sciences may begin the formulation development work for its clients even sooner. This enables projects to start more quickly and turnaround times to be decreased, which will shorten development timeframes. As a result, Quotient Sciences can swiftly transition drug programs downstream into GMP clinical trial manufacturing at the appropriate time, saving its customers valuable time in advancing cutting-edge medicines into the clinic. It is also important to note that the new equipment and process trains are fully integrated with the company’s existing capabilities.
The CEO of Quotient Sciences, Mark Egerton, stressed that the company’s goal is to assist patients receive innovative medications more quickly. For the past ten years, Quotient Sciences has been investing in the necessary infrastructure to support this goal. Supporting customer demand is extremely important to all of Quotient Sciences‘ stakeholders, and they are all committed to continuing their active efforts to expand the business’ formulation toolkit in order to offer more integrated solutions through a single organization and simplify the outsourcing paradigm for Quotient Sciences‘ clients.
About Quotient Sciences
A medication development and manufacturing accelerator, Quotient Sciences offers integrated programs and specialized services throughout the full development route. Quotient Sciences breaks through silos across a variety of drug development capabilities, saving valuable time and money in the process of getting medicines to patients. The company’s unwavering conviction that ideas must be transformed into solutions—specifically, that molecules must quickly become treatments—drives everything it does for clients. This is so that humanity can quickly find solutions. With over 1,300 employees, Quotient Sciences runs modern production and clinical facilities in the US and UK.
The employees are what make Quotient Sciences a unique place to work. To assist its clients in creating innovative medications for sick patients, the business is made up of a passionate team of committed individuals. Those that are dedicated to making a difference, excel at providing excellent customer service, and are always willing to go above and beyond are sought after by Quotient Sciences. Quotient Sciences‘ culture and profitability depend on teamwork; helping, supporting, and mentoring are ingrained in the fabric of the business.
Engr. Dex Marco Tiu Guibelondo, BS Pharm, RPh, BS CpE
Editor-in-Chief, PharmaFEATURES
Expect to join an innovative and empowering culture at Quotient Sciences, where daily chances for learning and growth are abundant and continual improvement is encouraged.

Learn more about Quotient Sciences on quotientsciences.com.
Subscribe
to get our
LATEST NEWS
Related Posts

Molecular Biology & Biotechnology
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.

Drug Discovery Biology
Unlocking GPCR Mysteries: How Surface Plasmon Resonance Fragment Screening Revolutionizes Drug Discovery for Membrane Proteins
Surface plasmon resonance has emerged as a cornerstone of fragment-based drug discovery, particularly for GPCRs.