In the dynamic realm of regenerative medicine, Alder Therapeutics emerges as a beacon of innovation, set to redefine the landscape of cell therapy development. As a preclinical phase stem cell therapy company, Alder positions itself as a transformative force, steering away from conventional methods to embrace a reduced-risk strategy that places commercial considerations at the forefront of its developmental programs. This groundbreaking approach marks a pivotal moment in the pursuit of effective treatments for diseases with high unmet needs.
Navigating the Challenges of Inherited Blindness
Retinitis Pigmentosa (RP), a rare group of genetic eye diseases, has long posed a daunting challenge to the medical community. Alder Therapeutics, however, is poised to tackle this obstacle head-on with its lead program—a mutation-agnostic cell therapy designed to treat RP. Unlike existing treatments limited to specific gene mutations, Alder’s retinal therapy holds the promise of being curative for the majority of RP patients. This revolutionary approach not only broadens the scope of eligible recipients but also streamlines manufacturing processes, making it a highly scalable and efficient solution.
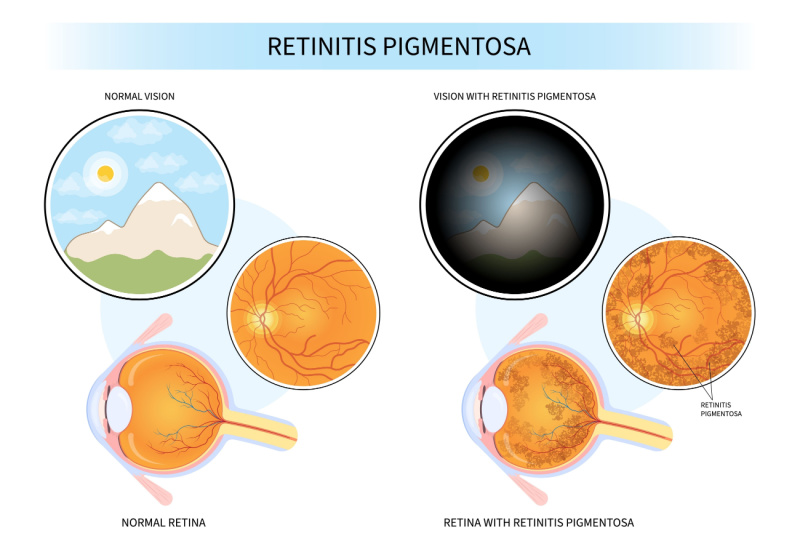
The CEO of Alder Therapeutics, Kristian Tryggvason, Ph.D., MBA, underscores the importance of their innovative approach to retinitis pigmentosa (RP) treatment. In contrast to current RP treatments confined to single-mutation gene therapy, limiting eligibility to a small fraction of patients, Alder’s retinal therapy is characterized as mutation-agnostic and potentially curative. This distinction suggests suitability for the majority of RP patients, marking a significant advancement in addressing the limitations of existing therapies.

Pluripotent Stem Cells: The Engine of Regeneration
At the heart of Alder’s transformative approach lies the utilization of pluripotent stem cells (PSCs), a potent cell type with the ability to give rise to various cell and tissue types needed for self-repair. PSC therapies, including Alder’s pioneering programs, open new avenues of hope for patients facing incurable diseases. While the potential of PSCs in treating various conditions, from diabetes to spinal cord injuries, is immense, traditional approaches to bringing such therapies to market are fraught with challenges.
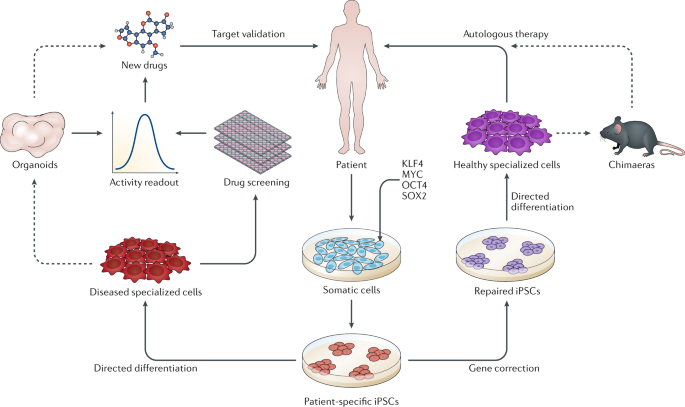
However, in 2022, Alder Therapeutics emerged as a game-changer, committed to overcoming these obstacles and unlocking the full potential of PSCs. The company’s approach not only prioritizes risk reduction but also integrates commercial viability assessments from the outset, promising a more reliable and streamlined path to success.
The Alder Approach: Reducing Risks, Enhancing Viability
Ricardo Baptista, Ph.D., serving as the Chief Technology Officer at Alder Therapeutics, provides insights into the company’s unique philosophy regarding cell therapy development. According to Baptista, Alder has formulated a novel approach that involves the creation of robust and scalable manufacturing processes from the outset. The company places emphasis on selecting suitable starting cell material and conducting commercial viability assessments to estimate return on investment (ROI). Additionally, Alder employs a virtual and agile operational structure, allowing them to leverage the expertise of the best professionals in propelling their initiatives forward. This distinctive methodology is anticipated to significantly mitigate risks along the path to achieving success in the field of cell therapy.

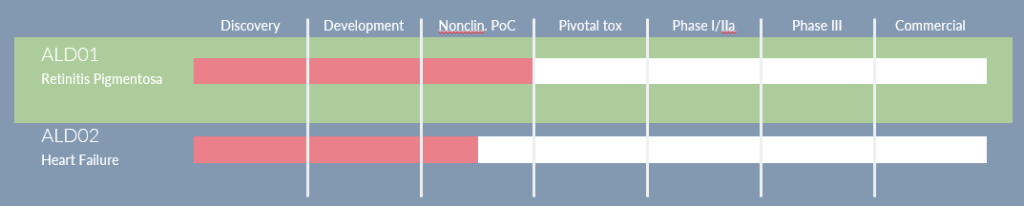
Alder Therapeutics has successfully secured €3m in seed funding, a testament to the promise presented by its RP and cardiovascular programs. With a commitment to progress the RP orphan drug program through Phase I/IIa by 2026 and prepare its cardiac program for GLP toxicity studies, the company aims to raise €22m. The stage is set for Alder Therapeutics to lead the charge in reshaping the cell therapy sector, offering hope to patients worldwide through innovative and risk-reduced treatments.
About Alder Therapeutics
Alder Therapeutics, propelled by a mission to cure the incurable, stands at the forefront of transformative pluripotent stem cell (PSC) therapies. Officially established in 2022, the seeds of Alder’s vision were planted a decade prior, inspired by co-founder Kristian Tryggvason’s discussions with his father, Professor Karl Tryggvason, and a belief in the untapped potential of laminin technology.
The pivotal moment arrived in 2022 when animal studies affirmed the benefits of photoreceptor cell differentiation and cardiac cell differentiation methods, developed by Professor Tryggvason with Duke-NUS’s support. Alder Therapeutics emerged, attracting attention from Linc and Flerie. In early 2023, the team licensed technology from Duke-NUS, successfully transferred manufacturing processes and introduced innovative analytical methods.
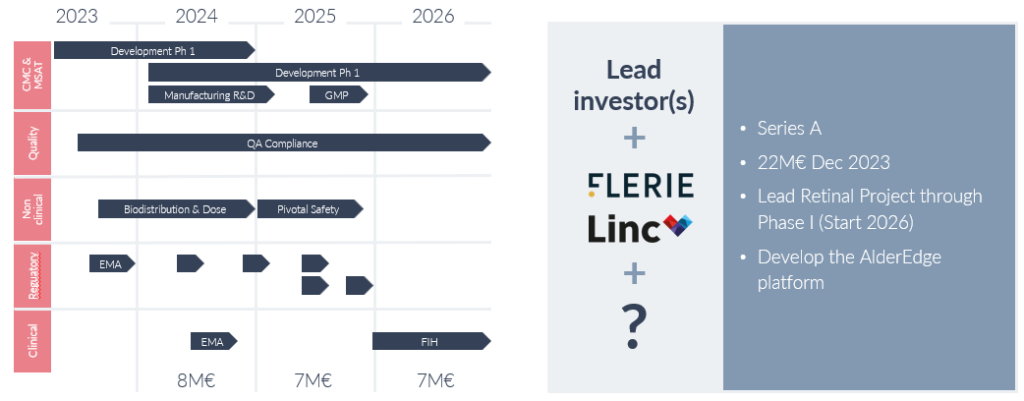
With an ambitious aim to secure funding in September 2023 for the groundbreaking ALD01 eye project’s Phase I, Alder Therapeutics looks ahead to 2024 for verification studies and GLP Tox studies, setting the stage for a landmark clinical trial in 2026. Beyond milestones, the journey reflects dedication to medical innovation, patient outcomes, and an unwavering commitment to curing the incurable.
Alder Therapeutics adopts the AlderEdge philosophy, a unique approach leveraging academic and commercial cell therapy experience. The AlderEdge principles aim to de-risk cell therapy development by considering critical determinants early on: manufacturing, safety and efficacy, commercial viability, and operational agility. This approach streamlines development and manufacturing, maximizing program success chances, and benefitting both patients and investors alike.
Learn more about the company from aldertx.com.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Cell & Gene Therapy
Eyeing the Future: Stem Cells and the Promise of Corneal Restoration
By reducing dependency on donor tissues and minimizing immunosuppressive demands, iCEPS has the potential to redefine LSCD treatment.
Cell & Gene Therapy
A Cure in the Code: How Gene Editing is Transforming the Fight Against Sickle Cell Disease
BIVV003’s success highlights gene editing’s potential to treat genetic disorders at their root, offering hope for other conditions.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.