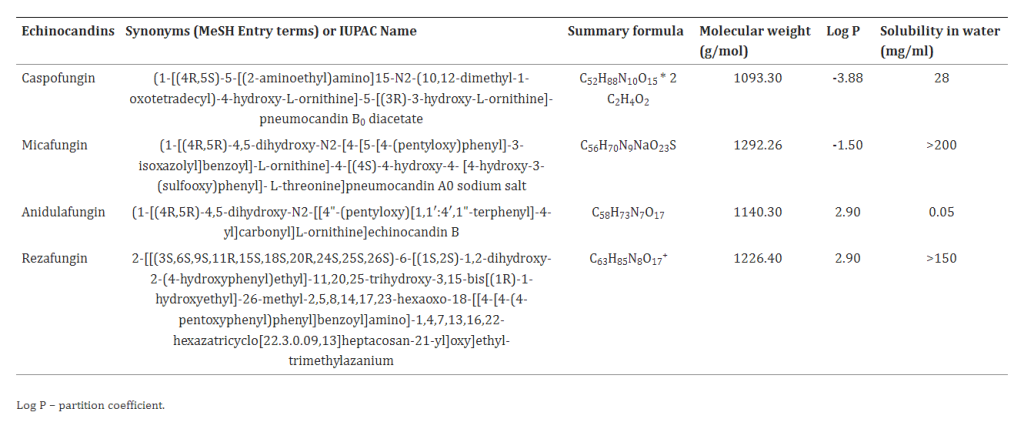
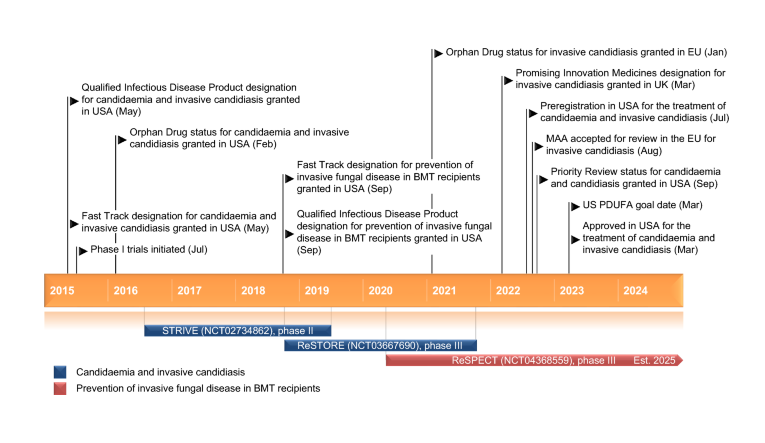
In a groundbreaking development, Mundipharma has announced the approval of rezafungin (rezafungin acetate, Rezzayo™) by the European Commission (EC) for the treatment of invasive candidiasis in adults. This monumental decision comes on the heels of a positive recommendation from the Committee for Medicinal Products for Human Use (CHMP), heralding a new era in antifungal therapy. The approval is grounded in the robust findings of the ReSTORE Phase III clinical trial, where rezafungin, administered once weekly, demonstrated statistical non-inferiority to the current standard of care, caspofungin, given once daily.
Revolutionizing Treatment Paradigms
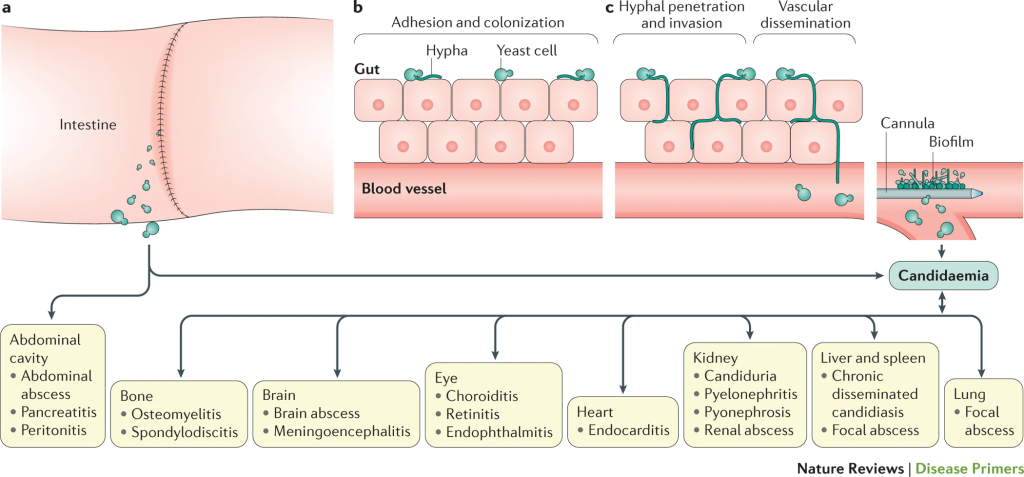
Invasive candidiasis, a severe and potentially life-threatening infection of the bloodstream and/or deep tissues, has long posed a significant challenge to healthcare professionals. The mortality rate, particularly among immunocompromised individuals, can soar to 40% or more, underscoring the urgency for innovative therapeutic solutions. The burden on healthcare systems is palpable, with prolonged treatment regimens and extended hospital stays amplifying the need for alternative approaches. Remarkably, the last 15 years witnessed a stagnation in treatment options, highlighting the critical necessity for a paradigm shift.
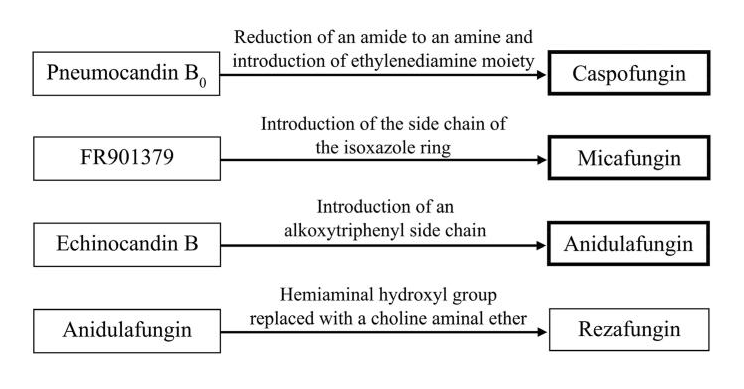
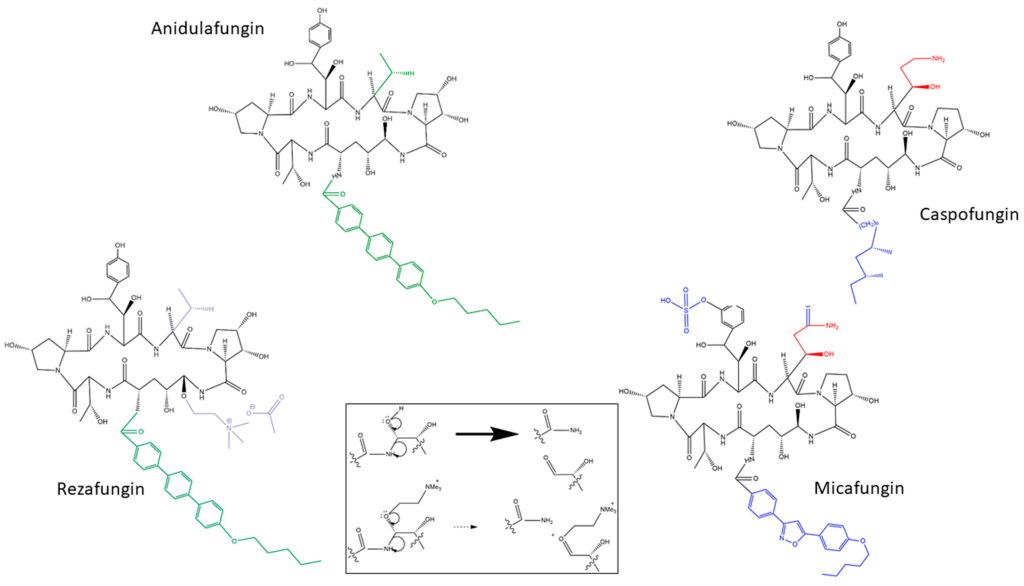
The STRIVE Phase II clinical trial and an exhaustive nonclinical development program buttress the ReSTORE trial’s findings, fortifying the case for rezafungin’s efficacy and safety. These trials collectively illuminate the potential of rezafungin to alter the trajectory of invasive candidiasis treatment.
STRIVE Phase II Clinical Trial
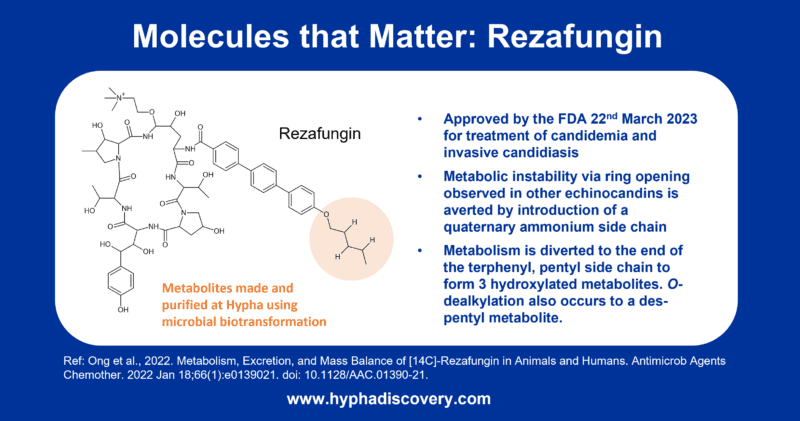
Rezafungin (RZF), hailed as a next-generation echinocandin, boasts notable pharmacokinetic advantages, characterized by a prolonged half-life (~133 hours) and elevated plasma drug concentrations early in the therapeutic course. This unique profile facilitates front-loaded, extended-interval dosing, a significant departure from conventional antifungal approaches. The pharmacokinetics of RZF exhibit dose-proportional behavior, showcasing minimal interpatient variability and a favorable safety profile. Preclinical studies further underline RZF’s efficacy in diverse settings, including animal models of candidemia, invasive candidiasis (IC), and Candida biofilms. Additionally, phase 1 trials in healthy volunteers have attested to the safety and tolerability of RZF.
The STRIVE trial (ClinicalTrials.gov, NCT02734862), constituting a pivotal phase 2 study, employed a rigorous design to compare the safety and efficacy of RZF administered once weekly (QWk) with caspofungin (CAS) given once daily for the treatment of candidemia and/or invasive candidiasis. Executed across 44 centers in 10 countries, STRIVE adhered meticulously to prevailing country and local regulations, the International Conference on Harmonisation Good Clinical Practice, and the Declaration of Helsinki. Ethical approval from ethics committees or institutional review boards was obtained, and written informed consent was procured from all participating patients.
The trial’s eligibility criteria encompassed male and female patients aged 18 years or older, exhibiting systemic signs of infection and mycological evidence of candidemia and/or IC within 96 hours before randomization. Exclusion criteria were defined to exclude certain forms of IC, Candida infections of the eye or central nervous system, neutropenia, severe hepatic impairment, and prior systemic antifungal therapy exceeding 48 hours.
The primary efficacy outcome centered on the overall response, with an overall cure defined as the resolution of clinical signs of candidemia/IC coupled with mycological eradication at day 14 (±1 day). Secondary efficacy outcomes included responses at various time points, mycological eradication definitions, and all-cause mortality at day 30. Safety assessments encompassed adverse events, treatment-emergent adverse events, all-cause mortality through follow-up, and vital signs, laboratory, and EKG testing.
In summary, the multicenter phase 2 trial, STRIVE, has successfully demonstrated the safety, tolerability, and efficacy of once-weekly RZF compared to once-daily CAS in the treatment of invasive candidiasis and candidemia. These encouraging findings support the feasibility of a once-weekly dosing strategy with RZF, laying the groundwork for further investigation in the succeeding phase 3 clinical trial, ReSTORE (NCT03667690), which directly compares once-weekly RZF (400/200 mg) to CAS in the treatment of candidemia and invasive candidiasis.
ReSTORE Phase III Clinical Trial
The ReSTORE trial, a multicenter, double-blind, double-dummy, randomized Phase 3 study, was conducted at 66 tertiary care centers across 15 countries. Inclusion criteria comprised adults (≥18 years) exhibiting systemic signs and mycological confirmation of candidaemia or invasive candidiasis. Participants were randomly assigned in a 1:1 ratio to receive either intravenous rezafungin once a week (initial 400 mg in week 1, followed by 200 mg weekly, totaling two to four doses) or intravenous caspofungin (70 mg loading dose on day 1, followed by 50 mg daily) for a maximum of four weeks.
The primary endpoints for evaluation were global cure, defined as the amalgamation of clinical cure, radiological cure, and mycological eradication at day 14 for the European Medicines Agency (EMA). Simultaneously, the 30-day all-cause mortality for the U.S. Food and Drug Administration (FDA) was assessed, both with a targeted non-inferiority margin of 20%. Assessment was conducted in the modified intention-to-treat population, encompassing all patients who received one or more doses of the study drug and had documented Candida infection based on a culture from blood or another normally sterile site obtained within 96 hours before randomization. Safety parameters were scrutinized by analyzing the incidence and types of adverse events and deaths in the safety population, defined as all patients who received any amount of the study drug.
From October 12, 2018, to August 29, 2021, a total of 222 patients underwent screening for participation, resulting in the random assignment of 199 patients. Among them, 100 were allocated to the rezafungin group, and 99 were assigned to the caspofungin group. In terms of demographics, 118 (59%) were men, 81 (41%) were women, with a mean age of 61 years (SD 15·2).
By day 14, 55 (59%) of 93 patients in the rezafungin group and 57 (61%) of 94 patients in the caspofungin group achieved a global cure, meeting the EMA primary endpoint. Regarding the FDA primary endpoint of 30-day all-cause mortality, 22 (24%) of 93 patients in the rezafungin group and 20 (21%) of 94 patients in the caspofungin group died or had an unknown survival status at day 30.

In the safety analysis, 89 (91%) of 98 patients in the rezafungin group and 83 (85%) of 98 patients in the caspofungin group experienced at least one treatment-emergent adverse event. Noteworthy treatment-emergent adverse events, occurring in at least 5% of patients in either group, included pyrexia, hypokalaemia, pneumonia, septic shock, and anaemia. Serious adverse events were reported in 55 (56%) patients in the rezafungin group and 52 (53%) patients in the caspofungin group.
Overall, the data indicate that rezafungin was non-inferior to caspofungin for the primary endpoints of day-14 global cure (EMA) and 30-day all-cause mortality (FDA). Further assessment of efficacy in the initial treatment phase is warranted. Notably, there were no alarming trends in treatment-emergent or serious adverse events. These phase 3 results affirm the efficacy and safety of rezafungin, providing robust support for its ongoing development.
The trial, registered with ClinicalTrials.gov under NCT03667690, has been completed, marking a significant milestone in the comprehensive evaluation of rezafungin’s efficacy and safety in treating invasive candidiasis.
A Beacon of Hope: Perspectives from Key Figures
Professor Oliver Cornely, Head of the European Excellence Centre for Medical Mycology at the University Hospital Cologne, Germany, and a crucial member of the Data Review Committee in the Phase III ReSTORE trial, voiced the significance of this milestone. In his commentary, he noted there exists a substantial global unmet need for addressing individuals with invasive candidiasis. The recent declaration by the European Commission represents a crucial juncture that has the potential to empower the healthcare professional community to navigate invasive candidiasis patients with a fresh approach, leveraging a novel treatment option.
Yuri Martina, Chief Development and Medical Officer at Mundipharma, expressed pride in the achievement, stating that the European approval is a culmination of years of developing an additional treatment option for invasive candidiasis patients and underscores their commitment to supporting the management of infectious diseases.
Orphan Status: Paving the Way for a Targeted Approach
In recognition of its potential impact, rezafungin has been granted Orphan Drug Designation for the treatment of invasive candidiasis in the EU. This designation reflects the unique status of the drug, acknowledging its potential to address a critical unmet need. The approval and orphan status together herald a new chapter in the fight against invasive candidiasis, providing a glimmer of hope for patients and clinicians alike.
The approval of rezafungin in the European Union is not merely a regulatory triumph; it represents a beacon of hope for individuals grappling with invasive candidiasis. This breakthrough underscores the commitment of the scientific community to push the boundaries of medical innovation, offering a promising alternative in the battle against a formidable adversary. As we celebrate this achievement, it sparks anticipation for a future where cutting-edge therapeutics redefine the landscape of infectious disease management.
About Cidara Therapeutics
Cidara Therapeutics, a biotechnology company headquartered in San Diego, California, utilizes its proprietary Cloudbreak® platform to develop drug-Fc conjugate (DFC) immunotherapies. These innovative therapies aim to save lives and enhance the standard of care for patients dealing with cancer and other severe diseases. The company focuses on creating targeted immunotherapies that inhibit specific disease targets while concurrently engaging the immune system, all facilitated by its proprietary Cloudbreak® platform.
Cidara is guided by core values such as collaboration, integrity, accountability, urgency, and courage, fostering a unique and award-winning atmosphere. This commitment underscores the company’s mission to develop groundbreaking immunotherapies with the potential to revolutionize patient care in the face of various critical illnesses.
Learn more about Cidara Therapeutics from their Official Website.
About Mundipharma
Mundipharma International Limited, a British multinational research-based pharmaceutical company, is owned by members of the Sackler family. The company has a global presence with locations in the United Kingdom, Canada, Germany, and Singapore. In Germany, Mundipharma operates as a subsidiary of both Mundipharma International Limited and Mundipharma AG, with its global headquarters situated at the Cambridge Science Park in Cambridge, England.
As a prominent global healthcare company, Mundipharma extends its reach across Africa, Asia Pacific, Canada, Europe, Latin America, and the Middle East. Specializing in Pain Management, Infectious Disease, Consumer Healthcare, and other severe and debilitating disease areas, Mundipharma is committed to delivering innovative treatments to patients.
Guided by principles anchored in Integrity and Patient-Centricity, Mundipharma places these values at the core of its operations. The company fosters a culture of inclusivity, continuous learning, and collaboration, encouraging its workforce to approach challenges with innovative thinking. This dedication to a patient-focused approach and an enriching work environment makes Mundipharma a distinguished and rewarding place to work.
For further information, please visit www.mundipharma.com.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Infectious Diseases & Vaccinology
Enduring Blockade: Five-Year Functional Antibody Persistence Against Emerging GII.4 and GII.17 Noroviruses
Natural infection with dominant GII noroviruses elicits long-lived functional antibodies, redefining immune durability in viral gastroenteritis.

Infectious Diseases & Vaccinology
Resistant Mycosis: Reimagining Antifungal Therapy Through Mechanistic Innovation and Molecular Disruption
Emerging antifungal strategies are redefining treatment by targeting fungal physiology through diversified, mechanism-driven approaches that overcome entrenched drug resistance.

Infectious Diseases & Vaccinology
Fungal Refractory States: Deciphering Pathobiology and Therapeutic Weaknesses in the Era of Rising Antifungal Resistance
Rising antifungal resistance reflects a multidimensional biological challenge that demands mechanistically innovative, host-integrated therapeutic strategies.

Infectious Diseases & Vaccinology
Epigenetic Parasite Vulnerabilities: Repurposing Histone Deacetylase Inhibitors for Complex Multispecies Therapeutics
Repurposed HDAC inhibitors target essential parasite epigenetic machinery, offering a powerful but complex frontier for next-generation antiparasitic therapy.
Read More Articles
Spatial Collapse: Pharmacologic Degradation of PDEδ to Disrupt Oncogenic KRAS Membrane Localization
PDEδ degradation disrupts KRAS membrane localization to collapse oncogenic signaling through spatial pharmacology rather than direct enzymatic inhibition.
Neumedics’ Integrated Innovation Model: Dr. Mark Nelson on Translating Drug Discovery into API Synthesis
Dr. Mark Nelson of Neumedics outlines how integrating medicinal chemistry with scalable API synthesis from the earliest design stages defines the next evolution of pharmaceutical development.
Zentalis Pharmaceuticals’ Clinical Strategy Architecture: Dr. Stalder on Data Foresight and Oncology Execution
Dr. Joseph Stalder of Zentalis Pharmaceuticals examines how predictive data integration and disciplined program governance are redefining the future of late-stage oncology development.
Exelixis Clinical Bioanalysis Leadership, Translational DMPK Craft, and the Kirkovsky Playbook
Senior Director Dr. Leo Kirkovsky brings a rare cross-modality perspective—spanning physical organic chemistry, clinical assay leadership, and ADC bioanalysis—to show how ADME mastery becomes the decision engine that turns complex drug systems into scalable oncology development programs.
Policy Ignition: How Institutional Experiments Become Durable Global Evidence for Pharmaceutical Access
Global pharmaceutical access improves when IP, payment, and real-world evidence systems are engineered as interoperable feedback loops rather than isolated reforms.







