The process of drug discovery has evolved from ancient times when natural compounds were used for medicinal purposes, to today’s high-tech methods like high-throughput screening, fragment-based screening, and virtual screening. As the landscape of drug development has advanced, a critical debate persists: Should identifying the molecular target of a drug and understanding its mechanism of action (MoA) be a priority early in the discovery process, or can these be delayed until later stages?
Some experts strongly advocate for early target identification (TID) and MoA elucidation, insisting that this information be gathered before human clinical trials commence. Others, however, argue that the discovery of the drug’s efficacy should take precedence, and that extensive focus on TID/MoA might divert crucial resources away from essential steps in drug development. This debate is not just academic; it has real-world implications for the pace and cost of drug discovery, particularly for complex diseases with no current treatment.
Target Identification vs. Phenotypic Screens: A Technical Overview
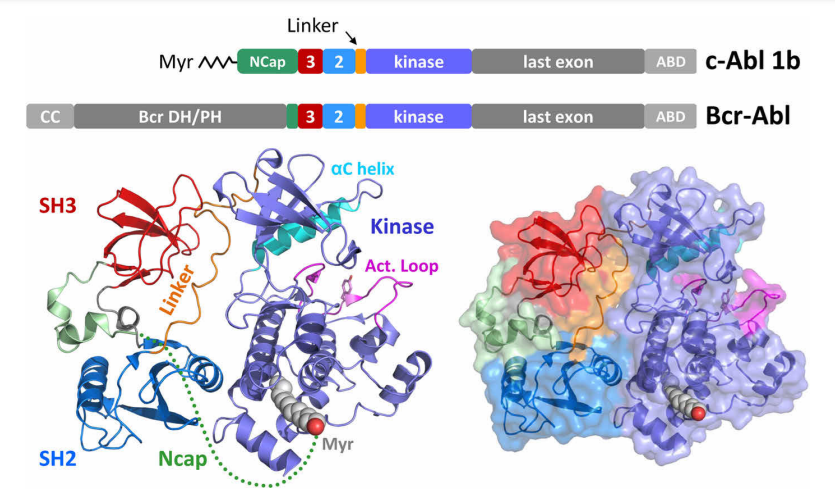
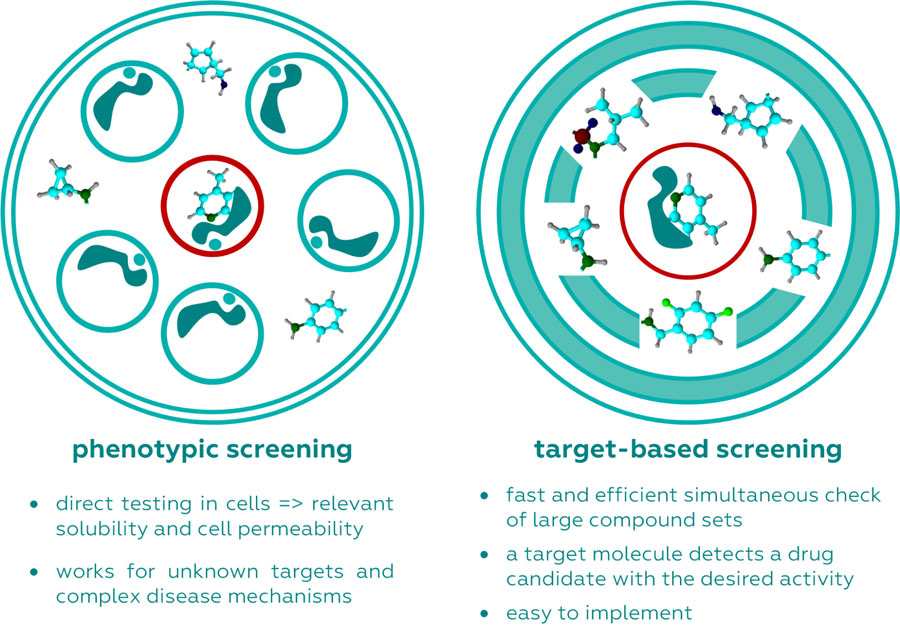
In the context of drug discovery, two main strategies have emerged: target-based screens and phenotypic screens. Target-based screens focus on identifying compounds that interact with a specific molecular target, often a protein involved in the disease process. These screens are highly efficient and cost-effective, with the advantage of high-throughput, rapid processing of compounds. Moreover, because these screens are informed by prior knowledge of disease biology, they allow for precise optimization of the drug’s activity, as seen in the development of drugs like imatinib for chronic myelogenous leukemia. In this case, target-based screening successfully identified the Abl tyrosine kinase as a key target, leading to the development of an effective therapy.
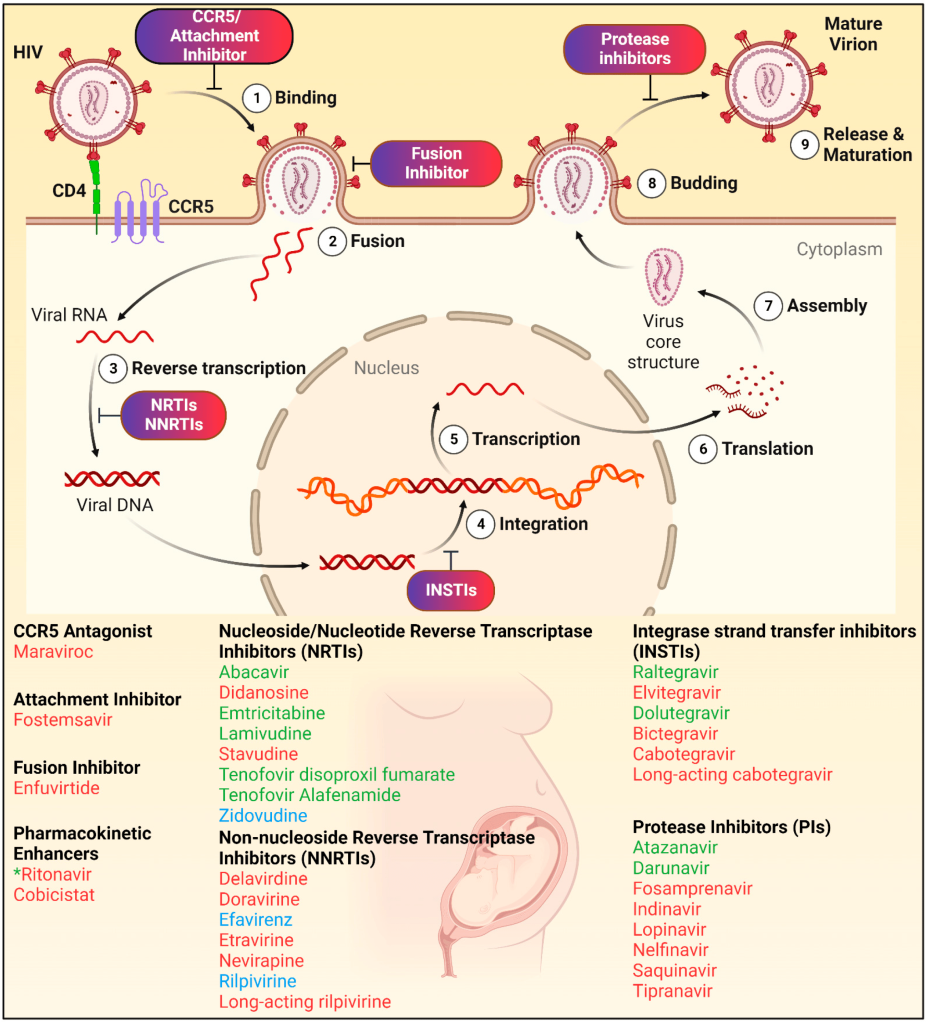
Another prime example of target-based screening is the development of HIV antiretroviral therapies. Early on, researchers identified key molecular targets essential for viral replication, such as reverse transcriptase and integrase enzymes. By focusing on these targets, small molecules were developed to inhibit the viral lifecycle, such as reverse transcriptase inhibitors (e.g., zidovudine) and integrase inhibitors (e.g., raltegravir). This precise targeting led to the creation of combination antiretroviral therapies (ART), which transformed HIV from a fatal condition into a manageable chronic disease, underscoring the value of target identification in drug discovery.
On the other hand, phenotypic screens offer a more holistic approach. Instead of focusing on a single molecular target, these screens observe the overall biological effect of a compound on cells, tissues, or even whole organisms. This approach mimics the biological complexity of living systems, offering insights into how a drug behaves in a more natural context. Phenotypic screening can sometimes uncover unexpected interactions or pathways that target-based approaches might miss, especially when the underlying disease mechanism is not well understood.
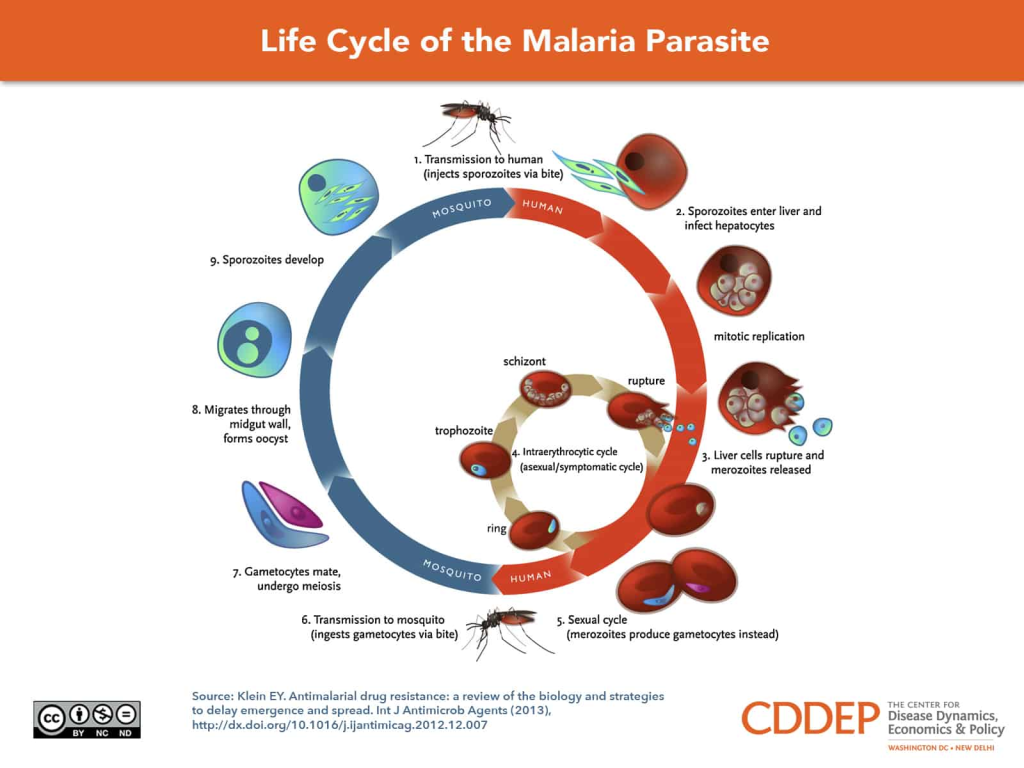
Phenotypic screening has been instrumental in the discovery of several important therapies, such as artemisinin for malaria. Artemisinin, derived from the sweet wormwood plant Artemisia annua, was identified through a traditional Chinese medicinal approach. However, it was the application of phenotypic screens that led to the understanding of its potent antimalarial properties. Instead of searching for a specific molecular target, researchers assessed the compound’s effects on Plasmodium parasites, which cause malaria, in infected red blood cells. This screening method revealed artemisinin’s ability to rapidly reduce parasite load, particularly during the early stages of infection, which was later found to be due to its action on heme and other parasite-specific factors. Importantly, this discovery occurred at a time when the molecular targets of malaria were poorly understood, highlighting the power of phenotypic approaches in cases where biological complexity defies simple target-based strategies.
Similarly, phenotypic screening is emerging as a crucial tool in the search for treatments for neurodegenerative diseases like Alzheimer’s, where the exact molecular mechanisms driving disease progression are still not fully elucidated. Alzheimer’s disease presents a multifaceted pathological landscape, involving amyloid-beta plaques, tau tangles, neuroinflammation, and synaptic dysfunction. Given this complexity, phenotypic screening offers a way to evaluate the overall impact of a compound on neuronal cells or animal models, without relying on an a priori hypothesis about the primary disease driver. For example, compounds that modulate synaptic activity or reduce neuroinflammation in a phenotypic context have shown promise, even if their precise molecular targets remain unidentified. This unbiased approach is particularly valuable in Alzheimer’s research, where previous attempts to target single molecules, such as amyloid-beta, have yielded limited therapeutic success. Phenotypic screens thus offer an opportunity to uncover novel therapeutic mechanisms and pathways that might be missed by conventional target-based approaches.
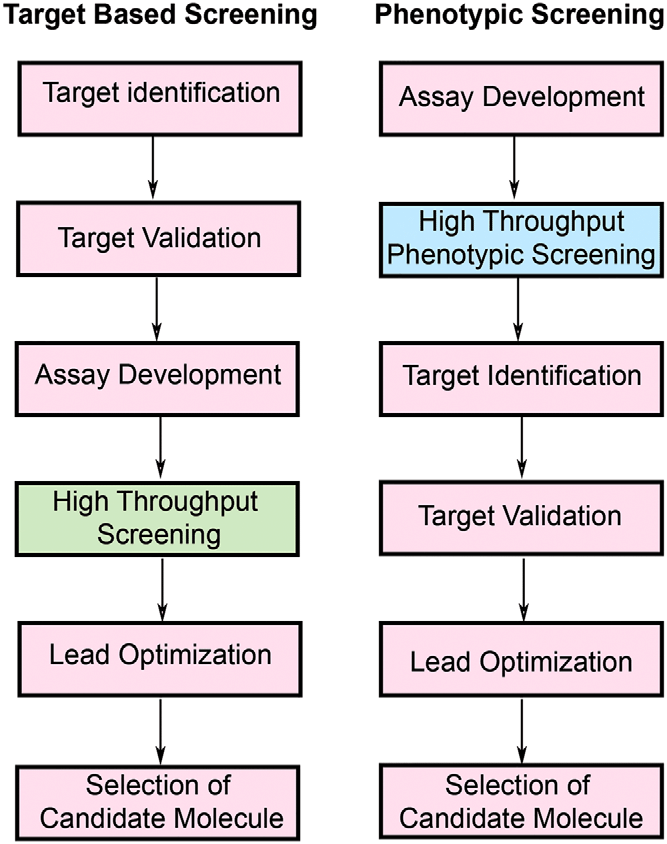
Advantages and Pitfalls of Target-Based Strategies
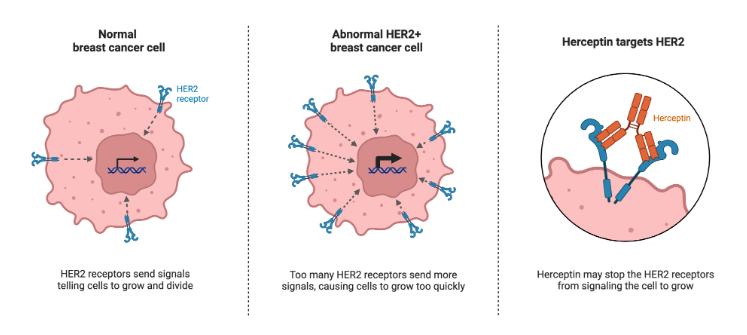
The main advantage of target-based strategies lies in their precision. By understanding the molecular target from the start, researchers can fine-tune the drug’s properties to maximize efficacy and minimize side effects. This approach has revolutionized personalized medicine. A prime example is trastuzumab, a drug used to treat HER2-positive breast cancer. The identification of HER2 as a target allowed for the development of a drug that directly addresses this form of cancer, improving survival rates significantly.
However, this precision comes with a downside: it requires a deep understanding of the disease’s molecular underpinnings. Without clear validation of the target, there is a risk that the drug may fail in clinical trials, as seen in many Alzheimer’s drug candidates. Even when a target appears promising, surprises can emerge. In recent studies, drugs believed to work by inhibiting specific cancer proteins were found to be effective even when those proteins were knocked out, suggesting that the drug’s success came from interactions with unknown targets.
This unpredictability highlights a core weakness of the target-based approach: it is only as good as the underlying knowledge of the disease. For many complex conditions, particularly neurological disorders, identifying an appropriate target can be exceedingly difficult. The lack of clear molecular targets has hampered efforts to develop new treatments for conditions like schizophrenia and bipolar disorder, where the interplay between genetics and environment creates layers of complexity.
The Phenotypic Approach: Broader, But Riskier
Phenotypic screening, by contrast, does not rely on prior knowledge of molecular targets. Instead, it examines the overall effect of a drug on a living system, capturing potential therapeutic benefits that may not be tied to a single molecular interaction. This approach is particularly useful for diseases where the pathophysiology is poorly understood. For instance, it played a key role in discovering drugs for tuberculosis and other infectious diseases, where the complex biology of the pathogen interacts with the host in ways that defy simple models.
However, phenotypic screens are not without their challenges. They tend to be less efficient and more resource-intensive than target-based approaches. Moreover, because these screens do not start with a specific molecular target, they often leave researchers with a question: what is the drug actually doing at the molecular level? This can make it harder to optimize the drug for human use and may increase the risk of unforeseen side effects. A notable example is lithium, which has been used for over a century to treat bipolar disorder, yet its precise mechanism of action remains a mystery. Despite extensive research, no clear molecular target for lithium has been identified, complicating efforts to develop more effective mood stabilizers.
When is Target Identification Necessary?
This brings us to the core of the debate: when should researchers prioritize target identification? Surprisingly, a drug’s mechanism of action is not always required for regulatory approval. In fact, many drugs currently on the market, including well-known ones like aspirin, were approved without a fully elucidated MoA. In cases where the therapeutic need is urgent, or the disease is poorly understood, it may make sense to focus first on proving the drug’s efficacy in clinical trials, with target identification following later.
For drugs being developed to treat conditions that already have some form of therapy, early target identification can add significant value. Knowing the molecular target allows for more precise drug optimization, leading to second- or third-generation therapies that are more effective and have fewer side effects. This has been the strategy behind the development of newer cancer therapies, where understanding the molecular drivers of the disease has led to more personalized, and often more effective, treatments.
On the other hand, for diseases with no effective treatment options—such as many neurological disorders—it may be more prudent to delay target identification until the drug has shown efficacy in preclinical models. Spending time and resources on target identification too early in the process could detract from the ultimate goal: finding a drug that works.
Balancing Scientific Rigor with Pragmatism
Ultimately, the decision of when to pursue target identification depends on the context of the disease being treated, the availability of existing therapies, and the resources at hand. Small biotech companies and academic researchers, who often work with limited resources, may find it more practical to push a drug forward without fully understanding its mechanism of action. In contrast, large pharmaceutical companies, with more resources and a greater risk of financial loss if a drug fails in clinical trials, may place a higher premium on target identification.
Regardless of the approach, it is clear that both target-based and phenotypic screens have their place in modern drug discovery. Rather than viewing them as opposing strategies, researchers should see them as complementary tools in the quest to develop new, life-saving therapies. By strategically employing both approaches, the field of drug discovery can continue to push the boundaries of science, ultimately delivering better treatments to patients in need.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts

Molecular Biology & Biotechnology
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.

Drug Discovery Biology
Unlocking GPCR Mysteries: How Surface Plasmon Resonance Fragment Screening Revolutionizes Drug Discovery for Membrane Proteins
Surface plasmon resonance has emerged as a cornerstone of fragment-based drug discovery, particularly for GPCRs.