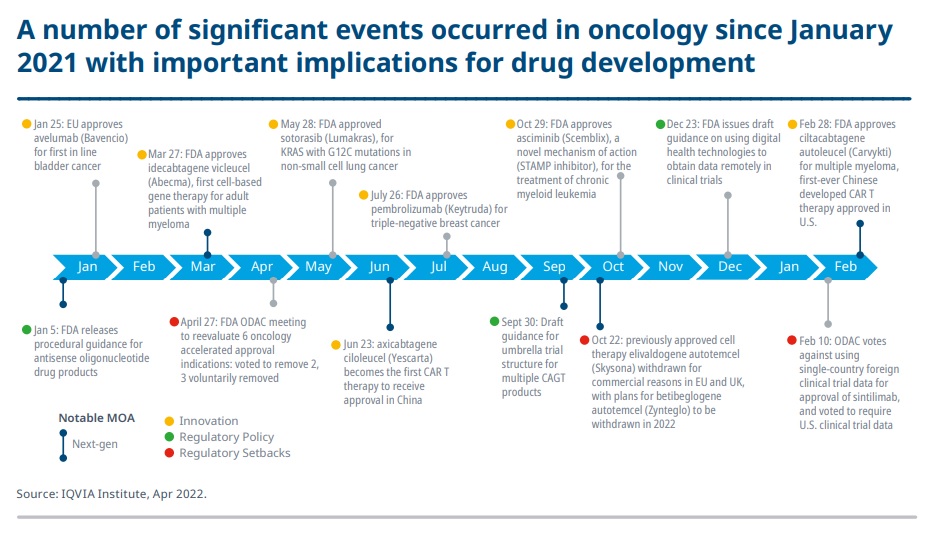
Newer oncological treatments made 2021 a monumental year in the fight against cancer. The number of cancer specific clinical trials peaked at 56% from 2016 with more focus on rare oncological disorders.
Cancer Treatment Status Quo
In IQVIA’s report, Global Oncology Trends 2022: Outlook to 2026 released last May 2022, cancer treatment expenditure is anticipated to surpass $300 billion by 2026. While the consolidated investment in novel diagnostics and drugs has demonstrated significant improvement in the clinical outcomes for millions of patients, the real concern remains – prolonged research and development (R&D) timelines.
Interestingly, streamlining of the clinical development process was pushed through in spite of pandemic restrictions. However, the ballooning complexity of clinical trials and skyrocketing administrative obligations in ensuring credibly-trained clinicians has been observably exacerbated by a drastic resource scarcity across the clinical research labor force.
In addition, while the Clinical tool has been adopted by some in the industry as a viable solution, the most recent Society for Clinical Research Sites (SCRS) annual survey revealed that the technology adds a mean 17.5 hours in training per study per site per month at a time when sites are thus far desperately short-staffed. Pair this up with rising complexity of cancer clinical trials and it is no wonder oncology is amongst the therapeutic areas with the lowest compounded success rates in 2021.
Labor Force Reskilling, A Solution to Modern Oncology Dilemmas
Patients are seldom diagnosed promptly enough for oncological study enrollment. This is the prime reason why early cancer detection proves to be a daunting task, as detailed in the research by the IQVIA Institute. Even the methodologies utilized in ensuring clinicians researching and espousing these advancing modalities prove to be outdated. Progressive technologies offered by decentralized clinical trials (DCTs), albeit helpful in cancer patient screening and enrollment and directing highly intricate rare cancer studies, are not the whole solution. Addressing foundational performance inadequacies and training gaps is the key. Significantly more well-equipped study team and clinicians are needed for significantly more complex study or treatment.
Together with major anticancer drug-developing companies, including Genentech, Regeneron, Daichi-Sankyo and others, ArcheMedX has shown a more efficient and effective performance improvement model to evaluate and skill or reskill over 5,500 clinicians on their unfolding therapies and treatments.
READY, The Surefire Power-Up Needed
A shocking 95% of the 5500+ oncology clinicians who participated in the on-demand activities of the ArcheMedX platform, READY, showed their lack of both needed knowledge and confidence in core competencies before undergoing more tailored clinical training on emerging therapies. This goes without saying that only the remaining 5% of the clinicians involved in oncology research and treatment showed ample expertise in making good diagnosis and correct treatment decisions.
The data generated by Ready revealed that this unfavorable number of clinicians were not requisitely prepared to genetically test cancer patients, screen them for early studies, and lacked the technical know-how and credence to choose the right treatment plan.
The aforementioned risks are easily abated via the provision of highly specialized and effectual education that betters preparedness in all key research and treatments objectives. After participation in ArcheMedX’s Ready-powered online training activities these clinicians favorably showed remarkable increase in knowledge and confidence. Clinicians augmented their key learning objectives mastery by more than 8x.

Statistics Does Not Lie
The on-demand training by ArcheMedX’s Ready platform produced an appropriately skilled clinical labor force with 87% more effectivity in cancer patients screening, 70% more competence in preventing and managing adverse drug events, and 56% more capability of identifying optimum oncology treatment plan.
Conversion of content into interactive, personalized learning experiences, analysis of how clinicians engage and behave during their learning, measurement of observable improvements in learner’s knowledge, confidence and behavior, provision of targeted remediation to clinicians on-demand, and clinician readiness assessment – these are the features of Ready that allowed attainment of such commendable performance impact.
Engr. Dex Marco Tiu Guibelondo, BS Pharm, RPh, BS CpE
Editor-in-Chief, PharmaFEATURES
Accelerate enrollment in your next study. Learn how Ready by ArcheMedX enables trial leaders to streamline the site initiation process and reveal which teams and sites are best prepared to successfully conduct your study. Request for a demonstration now by clicking the photograph below.

Web Media Acknowledgements
(1) Notable events in drug approvals and regulatory action image used in this article is adapted from Exhibit 1 of IQVIA’s institutional report entitled Global Oncology Trends 2022: Outlook to 2026. Institutional Report was published on May 26, 2022. The image was retrieved on November 10, 2022 from https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncology-trends-2022/exhibits/iqvia-institute-global-oncology-trends-2022-exhibit-1.png?mw=1440&hash=BDA725D6870420AB4DC9D4443AB703E1
(2) Oncology spending by region, US$Bn image used in this article is adapted from Exhibit 42 of IQVIA’s institutional report entitled Global Oncology Trends 2022: Outlook to 2026. Institutional Report was published on May 26, 2022. The image was retrieved on November 10, 2022 from https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncology-trends-2022/exhibits/iqvia-institute-global-oncology-trends-2022-exhibit-42.png?mw=1440&hash=FAD149105B400F3DF6DF9C4974B12614.
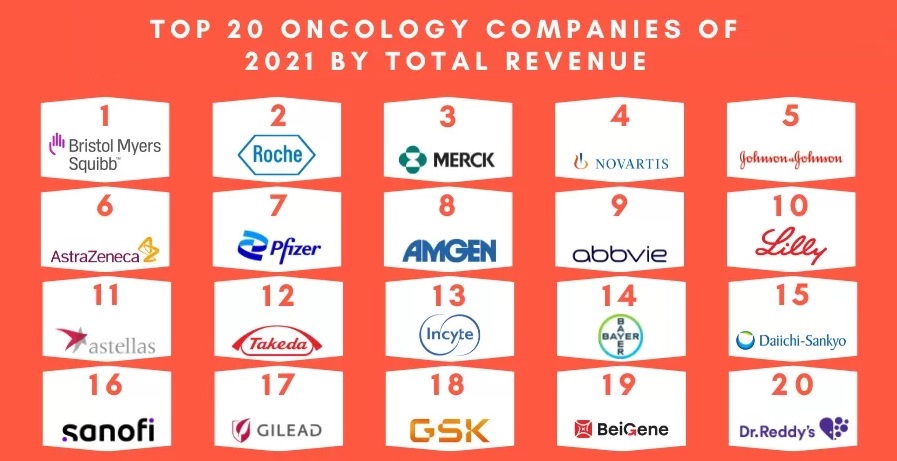
(3) List of the Top 20 Oncology companies based on their 2021 oncology segment revenue image used in this article is adapted from a PharmaShots article entitled Top 20 Oncology Companies Based on 2021 Oncology Segment Revenue. The article was authored by Shivani Chandra. Article was published on 08 Sept 2022. The image was retrieved on November 10, 2022 from https://pharmashots.com/8067/top-20-oncology-companies-based-on-2021-oncology-segment-revenue
(4) Ready, speeding up knowledge uptake image used in this article is adapted from a Facebook uploaded video entitled Ready by ArcheMedX Explainer Video. Video was uploaded on 21 Oct, 2020. The screencaptured image from the video was retrieved on November 10, 2022 from https://www.facebook.com/Archemedx/videos/355375299097645.
(5) The Ready Request a Demo image used in this article is adapted from an ArcheMedX article entitled AMA Recognizes ArcheMedX’s COVID-19 Report on Virtual Tech in Clinical Trials for Excellence. Article was published on 04 Oct, 2021. The image was retrieved on November 10, 2022 from https://www.archemedx.com/news/ama-honors-archemedx-report-virtual-tech-clinical-trials/.