If one thing is considered constant in industries involved in global healthcare and pharmaceutical manufacturing, it will always be the drive for greater insights of innovation and implementation of its novel technologies and methodologies. This is aimed to surmount major economic, or in this case, pandemic circumstances that conventionally require a highly-skilled, hence costly, human labor force.
Vanquishing COVID at Lightspeed
Pharmaceutical firms indubitably felt the need to do better than just doubling down on resource mobilization when COVID-19 reached its zenith. They realized better concerted efforts of execution on all levels of identification, development and trial must be mustered to generate a cornucopia of immunobiologicals and therapeutic modalities in hopes of curtailing the massive and menacing threat that SARS-CoV-2 posed.

Though undeniably catastrophic with both old and novel coronavirus strains ravaging huge chunks of the human population, the world felt safer with unassailable groundbreaking work from Pfizer/BioNTech, Moderna, Johnson & Johnson, and AstraZeneca. The vaccines and adjunct therapeutics (e.g., dexamethasone) were properly developed and responsibly and responsively distributed; thus, saving millions of lives.
Pandemic Protocols and Participants, Hurdles to Drug Development
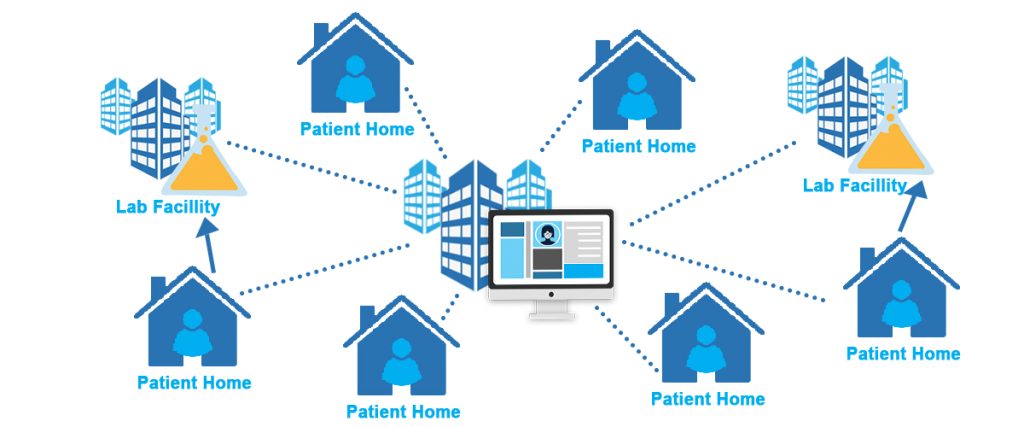
With a global collaboration set in place, a prime hurdle presents itself seeking to be solved – there is an observable inversely proportional relationship with the need for clinical trial participants vs the health care institutions available for accommodating such altruistic and humanity-enriching endeavors.
The aforementioned noticeable dilemma is exacerbated by the stringent protocols that must be held in place so as to restrict the movement in people in hopes of gaining some or better epidemiological control of viral spread. The obvious choice for most in the industry is to carry out decentralized clinical testing (DCTs), banking on technologies like virtual medicine and direct-to-patient (DtP) drug delivery.
A GlobalData research showed a significant 7% increase (18% in 2021) of trials adopting decentralization modalities from 2020’s 11%.
Leading Temperature-Controlled Logistics in Healthcare
CRYOPDP, being amongst the leading global providers of temperature-controlled logistics, plays a pivotal role in ensuring pharmaceutical and clinical trial communities can seamlessly perform decentralized and direct-to-patient trials during the pandemic.
Seen to be a temporary deviation from the norm, decentralization schemes implemented during the pandemic are here to stay. The Clinical Trials Arena-conducted program Decentralized Clinical Trial Adoption Tracker demonstrated a remarkable augmentation of remote drug delivery usage and home health and nursing care trials in 2021 with 50 newer trials embarked on.
2022 is set to experience improved support for the direct-to-patient methodology as it allows contract research organizations (CROs)/ trial sponsors to find novel opportunities and pioneering means of executing clinical trials that were highly underutilized pre-pandemic.
Balancing Speed and Execution

The velocity observed in streamlining the approval process is attributable to the modification implemented during the pandemic. Pre-pandemic timeline for securing trial approval reaches months and sometimes even years. The pandemic conveyed a huge need to accelerate the trial approval period. This is according to CRYOPDP’s chief operating officer (COO), Tangi Tremorin.
With the needed expedition came a price to pay, demanded in the form of intensive care and obligatory meticulousness so as to guarantee that the pursuit for greater approval speed did, in no way, circumvent and counterproductively compromise executional quality of clinical procedures and processes.
This is where CRYOPDP shines through and through. Its establishment of a suite of standard operating procedures (SOPs), checklists and clinical trial instructions across every level of the business secures timely drug delivery to clinical trial participants without trading off compliance to regulatory and data protocols.
Complexity is seen to rise with more stakeholders and heavier reliance on technological means for delivery tracking and information storage. Despite its entropic nature, excellent implementation of decentralized clinical trials, as proven by the pandemic, is something CRYOPDP visualizes as a major opportunity – a chance to lead, assist and expedite reliable temperature-controlled logistics better in the future.
Engr. Dex Marco Tiu Guibelondo, BS Pharm, RPh, BS CpE
Editor-in-Chief, PharmaFEATURES
CRYOPDP was voted as Pharma Leaders India’s Most Trusted Innovative Logistics & Supply Chain Company 2022 at the 15th Annual Pharma Leaders Power Brand Awards 2022. Click on the photo below to learn more about CRYOPDP, its mission and world-class logistics services.

Web Media Acknowledgements
(1) Title Background Featured Banner Image used in this article is adapted from an Ophthalmology Times article entitled Considering double-organ bias in clinical trials of eyelid ptosis. Photograph is taken from Adobe Stock Images repository. The article was authored by Lynda Charters. Article was published on 01 Sept 2021. The image was retrieved on November 10, 2022 from https://www.ophthalmologytimes.com/view/considering-double-organ-bias-in-clinical-trials-of-eyelid-ptosis.
(2) Decentralized Clinical Trials image used in this article is adapted from a Florence article entitled Five Things You Need to Know About Decentralized Clinical Trials Today. The article was authored by Blake Adams. The image was retrieved on November 10, 2022 from https://florencehc.com/learn/blog-posts/decentralized-clinical-trials-what-the-future-holds.
(3) Pfizer NYSE Bell Ringing image used in this article is adapted from a Forbes article entitled Pfizer And Moderna Rally After FDA Grants Full Approval To Pfizer’s Covid-19 Vaccine. The article was authored by Jonathan Ponciano. Article was published on 23 Aug 2021, 06:50pm EDT. The image was retrieved on November 10, 2022 from https://www.forbes.com/sites/jonathanponciano/2021/08/23/pfizer-and-moderna-rally-after-fda-grants-full-approval-to-pfizers-covid-19-vaccine/?sh=704ab5c6a222.
(4) Number of subjects in oncology clinical trials image used in this article is adapted from Exhibit 16 of IQVIA’s institutional report entitled Global Oncology Trends 2022: Outlook to 2026. Institutional Report was published on May 26, 2022. The image was retrieved on November 10, 2022 from https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncology-trends-2022/exhibits/iqvia-institute-global-oncology-trends-2022-exhibit-16.png?mw=1440&hash=E5CB021B7F14004212F127BAF45F0258.
(5) The Decentralized Clinical Trials Continuum image used in this article is adapted from a Veristat article entitled Understanding Decentralized Clinical Trials. The image was retrieved on November 10, 2022 from https://www.veristat.com/defining-decentralized-clinical-trials-audio-infographic.
(6) Direct-To-Patient Solutions for a Rare Disease Clinical Study image used in this article is adapted from a QuickSTAT case study entitled Direct-To-Patient Solutions for a Rare Disease Clinical Study. The image was retrieved on November 10, 2022 from https://quickstat.quick.aero/case-studies/direct-to-patient-logistics/.
(7) India’s Most Trusted Innovative Logistics and Supply Chain Company 2022 image used in this article is adapted from CRYOPDP’s Official Website. The image is found on CRYOPDP’s image slider on its homepage. The image was retrieved on November 10, 2022 from https://www.cryopdp.com/sites/cryopdp.com/files/styles/header_full/public/2022/09/12/news_award-01.png?h=9631399e&itok=XV8auiLr.