Throughout the process of drug development, encompassing diverse patient populations in clinical trials is crucial. This diversity aids in gathering comprehensive evidence on the safety and effectiveness of investigational medical products, ensuring their viability for a broad range of potential users upon approval. However, the establishment of eligibility criteria for clinical trials sometimes leads to the enrollment of cohorts that may not accurately represent the wider patient demographic.
In recent decades, there has been a concerted effort to address this issue through policy initiatives aimed at enhancing the inclusivity of clinical trials. These initiatives, spearheaded by entities such as the U.S. Food and Drug Administration (FDA) and the National Institutes of Health (NIH), specifically target underrepresented groups, such as women and older adults. The goal is to ensure that eligibility criteria are scientifically justified and encompass a more diverse patient population. Despite these strides, persistent challenges and barriers continue to impede full participation in clinical trials.
The Role of Inclusion and Exclusion Criteria
Clinical trials, the bedrock of evidence-based medicine, rely heavily on meticulously crafted eligibility criteria to define the study’s patient population. These criteria, often split into inclusion and exclusion categories, dictate who can participate, ensuring a controlled environment for assessing treatment efficacy. Inclusion criteria specify essential patient characteristics, such as disease stage or specific genetic markers, necessary for enrollment. Conversely, exclusion criteria delineate factors, like comorbidities or concurrent medications, that disqualify individuals from participation to mitigate confounding variables. However, the fine-tuning of these criteria can lead to a trade-off between precision and generalizability.
While stringent eligibility criteria minimize confounding variables, enhancing the probability of detecting treatment effects, they risk excluding subgroups that mirror real-world patient diversity. For instance, omitting individuals with comorbidities may overlook the treatment’s efficacy and safety in a prevalent patient subset. Therefore, a delicate balance must be struck between minimizing heterogeneity to ensure statistical robustness and capturing the breadth of patients likely to use the approved therapy.
Exclusion-Leading Ethical and Scientific Factors
Deciding clinical trial eligibility involves weighing risks and benefits. Concerns about adverse effects often exclude older adults, those with chronic conditions, and certain demographic groups like children and pregnant women. Yet, excluding them offers no insight into the drug’s effects post-approval. Hence, such exclusions must be evaluated case by case.
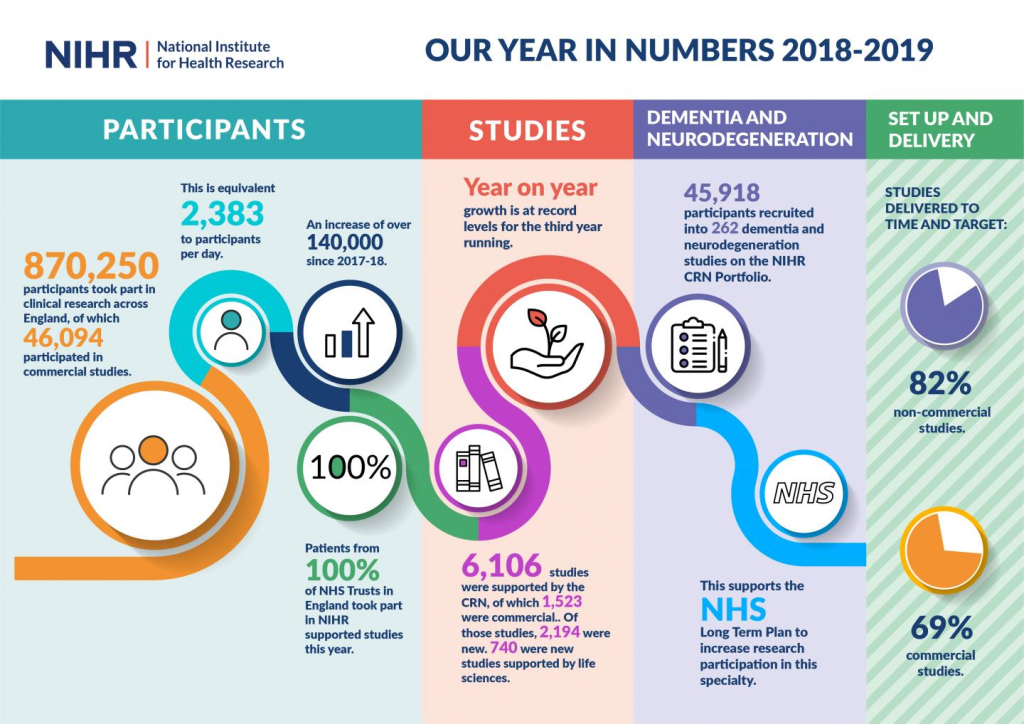
Navigating Clinical Trials Amidst Organ Impairments
Organ dysfunction, often denoted by markers like liver enzymes or renal function, frequently prompts exclusion from clinical trials. Such exclusions, prevalent in the pharmaceutical landscape, contribute to substantial evidence gaps concerning the product’s efficacy and safety in these cohorts. Despite potential dose adjustments, the nuanced responses in patients with organ pathologies elude scrutiny, leaving critical aspects of therapeutic outcomes uncharted.
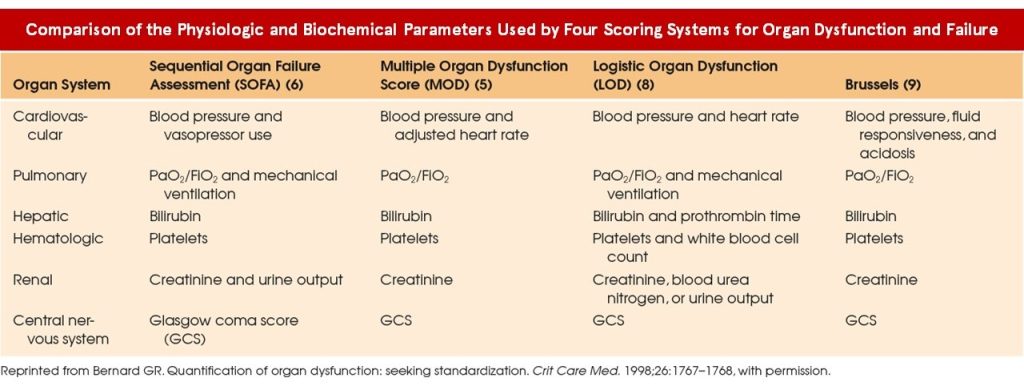

Unraveling the Complexity of Multimorbidity
Patients harboring multiple chronic conditions represent a demographic susceptible to exclusion due to heightened complexities. The intricate interplay between underlying morbidities and concomitant therapies poses challenges in ascertaining causality and delineating treatment effects. Yet, their omission hampers the trial’s representativeness, obscuring pivotal insights into real-world applications and therapeutic interactions.
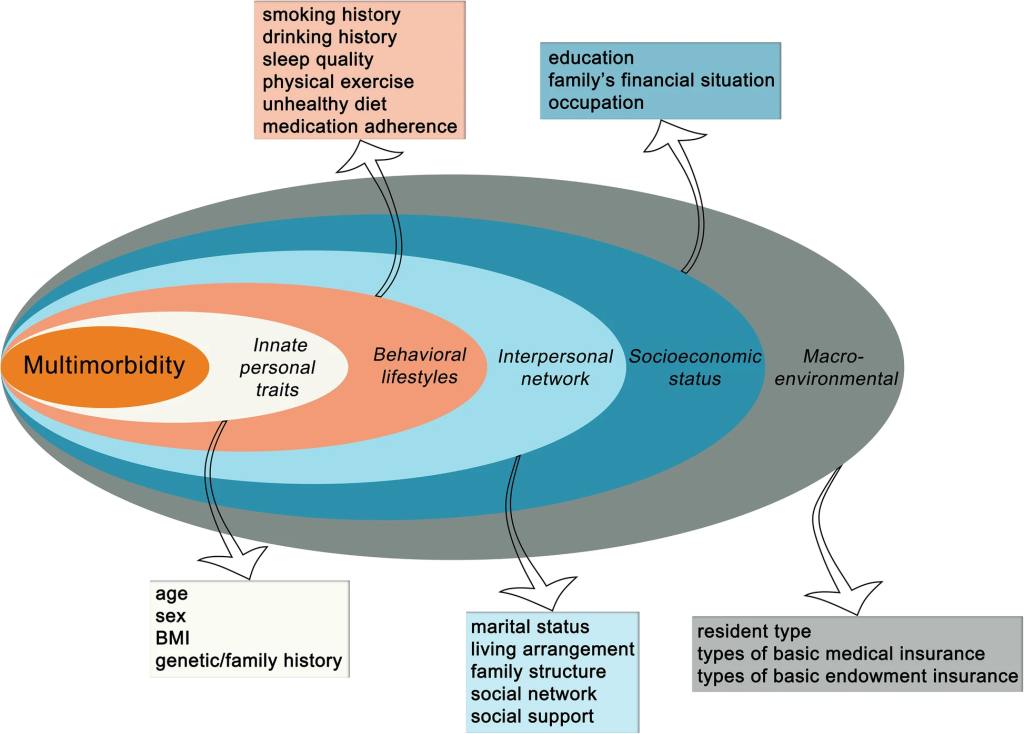
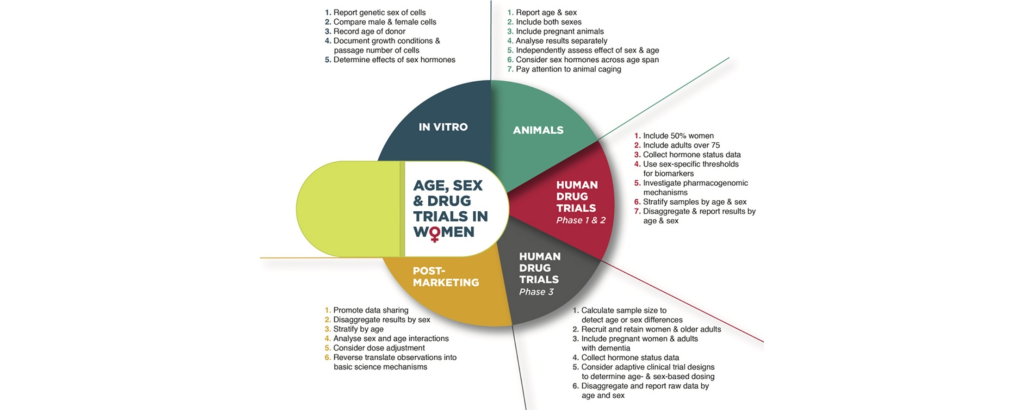
Bridging the Age Disparity in Clinical Trials
Despite their substantial representation in disease demographics, older adults confront notable underrepresentation in clinical trials. This demographic imbalance not only impedes the extrapolation of findings but also hinders informed decision-making regarding Medicare coverage. Recent legislative endeavors underscore the imperative of inclusivity, mandating concerted efforts to rectify age-related disparities and ensure equitable representation across age strata.

Pioneering Pediatric Inclusion
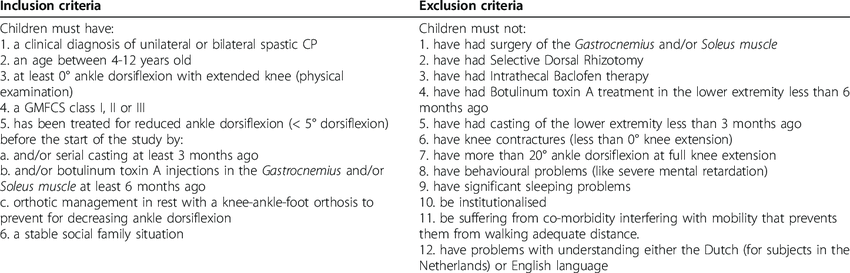
Historically, pediatric populations have encountered exclusionary practices, necessitating legislative interventions to rectify research disparities. Legislative frameworks, such as the BPCA and PREA, herald a paradigm shift by incentivizing pediatric inclusion and fostering collaborative endeavors to address pediatric therapeutic needs. Nonetheless, the intricacies of pediatric research necessitate tailored strategies to navigate consent processes and enhance enrollment endeavors.
Striving for Inclusivity Amidst Ethical Complexities
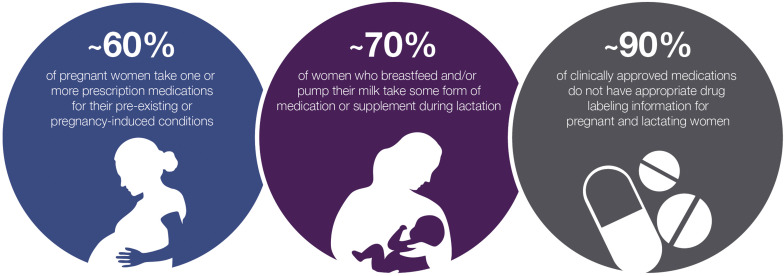
The exclusion of pregnant and lactating women stems from multifaceted ethical considerations, compounded by liability apprehensions and regulatory safeguards. Despite inherent complexities, burgeoning legislative initiatives and regulatory guidance underscore the imperative of inclusivity in this demographic. The formation of PRGLAC and issuance of FDA guidance herald pivotal strides towards addressing knowledge lacunae and ethical imperatives concerning maternal-fetal health.
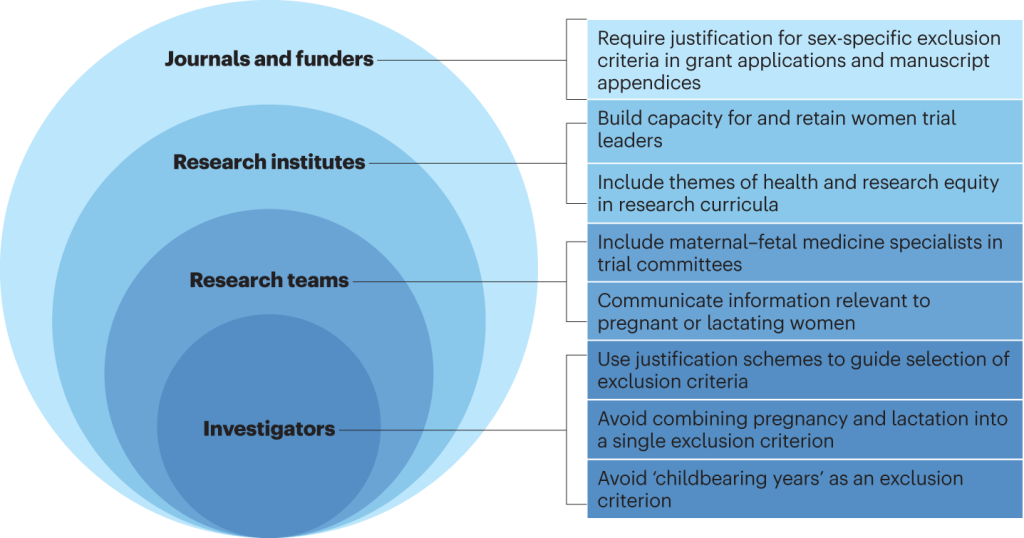
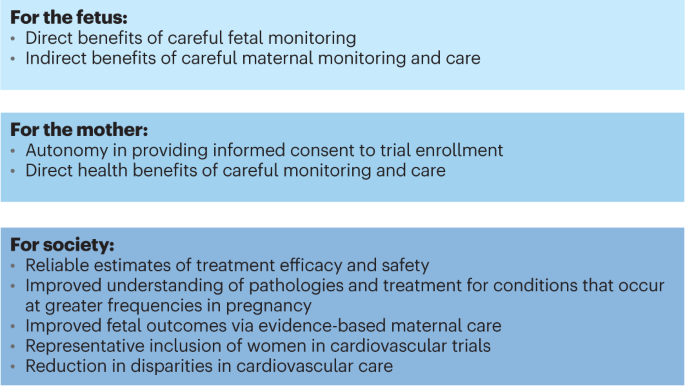
In essence, the pursuit of inclusivity in clinical trials transcends scientific imperatives, embodying a nexus of ethical, legislative, and public health considerations. As stakeholders converge to navigate these multifaceted challenges, collaborative endeavors and legislative mandates serve as beacons, illuminating a pathway toward equitable and comprehensive clinical research.
Link to FDA Public Workshop Report | The National Press Club (Washington, DC, April 16, 2018).
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts

Clinical Trial Supply Chain
From Chaos to Control: The Clinical Reinvention of Supply Chain through Data-Driven Infrastructure
In a healthcare landscape increasingly dominated by automation and AI, it’s tempting to see technology as a cure-all.
Clinical Trial Supply Chain
Deciphering the Nexus: Cluster Analysis in Shaping Regional Supply Chain Hubs
Cluster analysis has become instrumental in understanding and optimizing supply chains.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.













