In a groundbreaking development, Takara Bio Inc. has recently entered into a License Agreement with Fondazione Telethon ETS, an esteemed non-profit organization based in Italy. This strategic alliance empowers Telethon with a commercial license to leverage Takara Bio’s cutting-edge technology, RetroNectin®. This innovative method, rooted in Takara Bio’s proprietary RetroNectin® technology, holds immense promise in advancing the field of “Engineered Gene Therapy.”
The RetroNectin® Method: A Game-Changer in Gene Transduction
Enhancing Efficiency in Gene Transfer to Hematopoietic Cells
Research involving hematopoietic cells and suspension cells has historically faced challenges due to the low efficiency of gene transfer. RetroNectin® reagent, a recombinant human fibronectin fragment, emerges as a beacon of hope, facilitating the colocalization of lentivirus or retrovirus with target cells. This interaction results in a remarkable enhancement of transduction efficiency, positioning RetroNectin® reagent as the gold standard in transduction enhancers for retroviral/lentiviral gene transfer to hematopoietic cells. The reagent’s success is underscored by its extensive use in over 40 clinical trials, marking a significant stride in Engineered Gene Therapy.
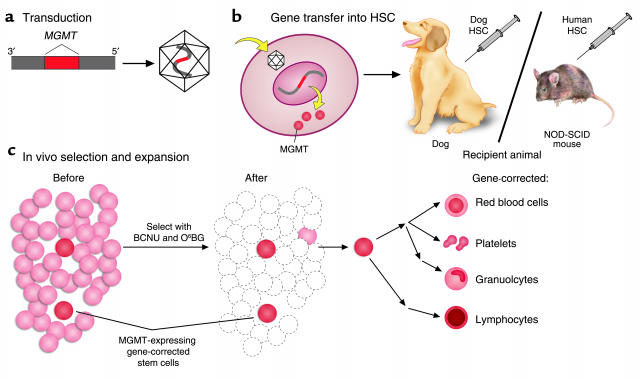
Unveiling the Mechanism: RetroNectin® Reagent in Action
RetroNectin® reagent’s effectiveness lies in its ability to promote the colocalization of viral particles and target cells, fostering a conducive environment for gene transduction. The interaction between virus particles and RetroNectin® reagent, mediated by the H-domain, coupled with the binding of target cells through integrin receptors VLA-5 and VLA-4, orchestrates a symphony of efficient gene transfer. Notably, VLA-4-expressing cells encompass a spectrum, including T cells, B cells, monocytes, and lymphoid progenitors, expanding the scope of RetroNectin’s applications.
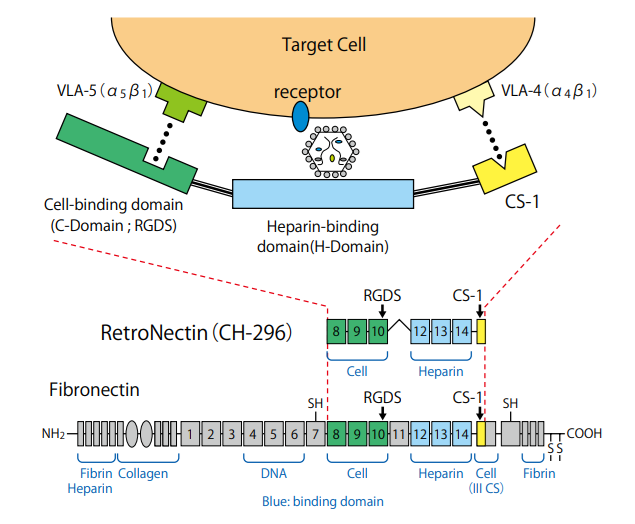
The cell binds to the CS-1 site via VLA-4, and to the C-domain via VLA-5. The
viral particle can bind to the H-domain of RetroNectin. These interactions
increase the localized concentrations of cells and viral particles, an effect that is
thought to enhance gene transduction. RetroNectin®
(Recombinant Human Fibronectin Fragment) Product Manual. TaKaRa Bio.
Beyond Gene Transfer: RetroNectin® Reagent’s Role in T-Cell Expansion
RetroNectin® reagent extends its prowess beyond gene transfer, playing a pivotal role in enhancing T-cell expansion. In the realm of in vitro expansion of CD8+ T cells from peripheral blood mononuclear cells, the presence of RetroNectin® reagent significantly boosts efficiency. Moreover, the resulting T-cell population exhibits a high proportion of naive T cells, underlining the multifaceted utility of RetroNectin® in Engineered Gene Therapy.
Navigating the Regulatory Landscape: RetroNectin® GMP Grade
As Engineered Gene Therapy progresses towards clinical applications, questions arise about the use of RetroNectin GMP grade. Takara Bio’s responses provide clarity, emphasizing the product’s suitability for research-based clinical trials and the necessity of regulatory approval for commercial use. Crucially, RetroNectin GMP grade’s registration in the Drug Master File (DMF) adds an additional layer of transparency, ensuring adherence to stringent quality standards.
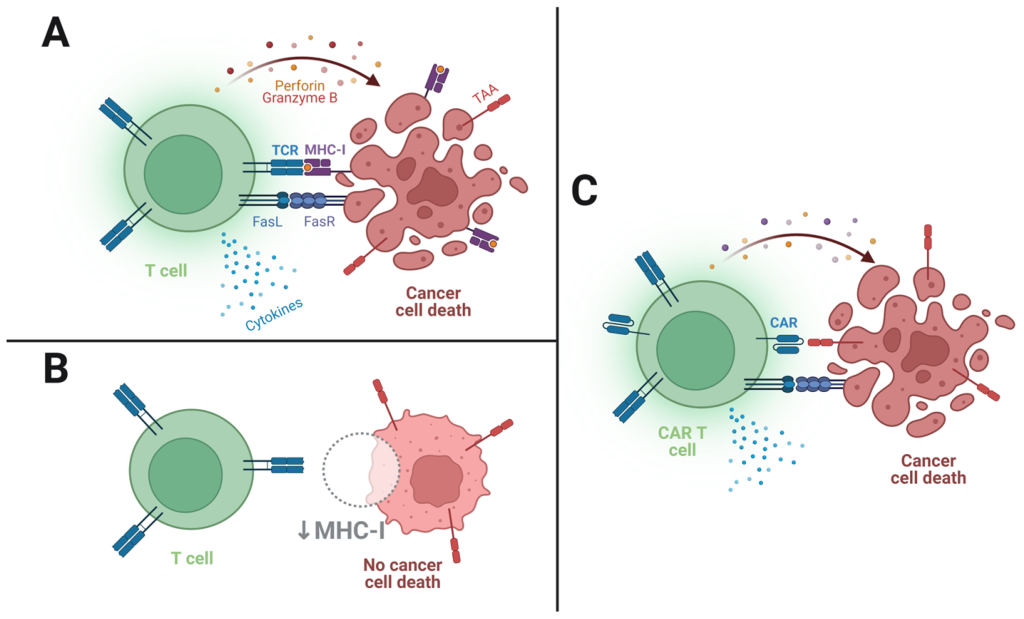
TCR & CAR T: Navigating the Future of Adoptive Cell Therapy
Precision in Targeting Tumor Markers
T-cell receptor (TCR)-based adoptive therapy emerges as a transformative approach, harnessing genetically modified lymphocytes against specific tumor markers. The intricate process involves patient screening, leukapheresis, TCR product generation, lymphodepletion, and therapy infusion. The delicate balance lies in selecting the right antigen and TCR for optimal efficacy, addressing challenges such as off-target effects and the diversity of peptide-MHC interactions. TCR-based ACT, overcoming barriers faced by traditional immunotherapies, ushers in a new era of precision in cancer treatment.
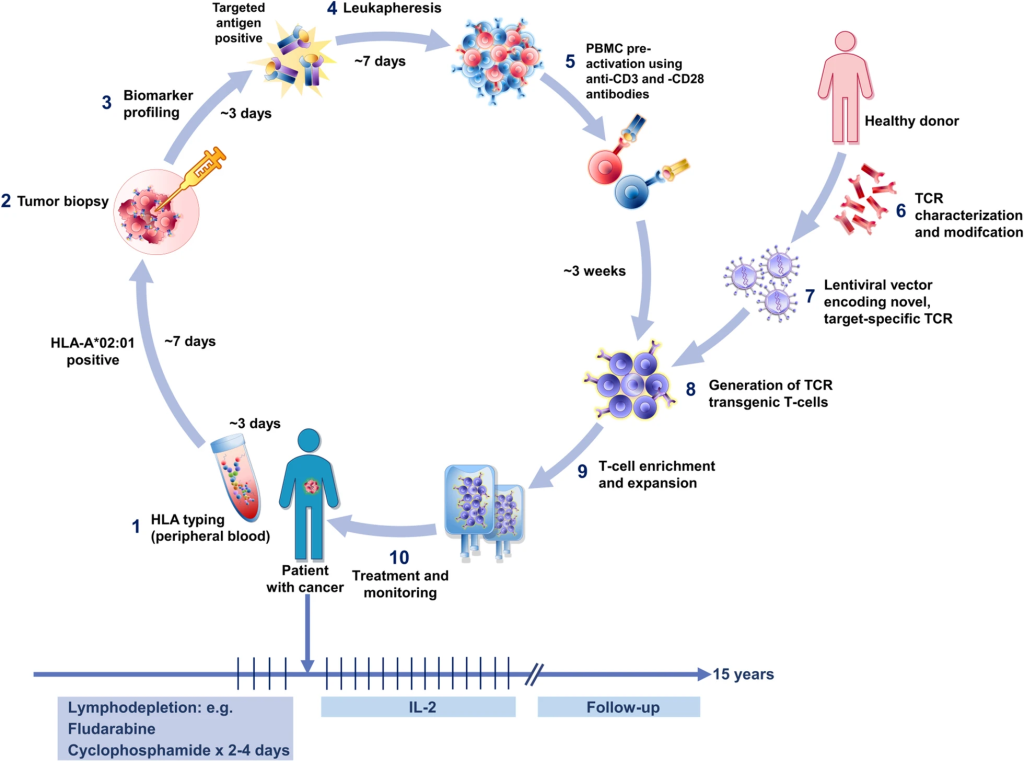
A Symphony of Precision and Versatility
Chimeric Antigen Receptor (CAR) T cells stand at the forefront of the Adoptive Cell Therapy era, marking a paradigm shift in oncological care. Engineered from a patient’s own lymphocytes, CAR T cells harbor a customizable CAR construct, offering unparalleled tunability in effector cell properties. The intricacies of CAR T cell activation and killing, though reminiscent of normal T cell receptor signaling, reveal nuanced differences that influence therapeutic outcomes. Notably, the modular composition of CARs allows for exquisite control over affinity, persistence, and potency, positioning them as a versatile tool in precision medicine.
Strategic Collaborations: Telethon’s Access to RetroNectin® for CGT
Advancing Rare Disease Treatment with Strimvelis
In an agreement with Takara Bio, Fondazione Telethon gains access to RetroNectin® for the production of cell & gene therapy products, including the groundbreaking Strimvelis. This rare disease treatment targeting adenosine deaminase deficiency – severe combined immunodeficiency (ADA-SCID) exemplifies the tangible impact of strategic collaborations in advancing the frontiers of Engineered Gene Therapy.
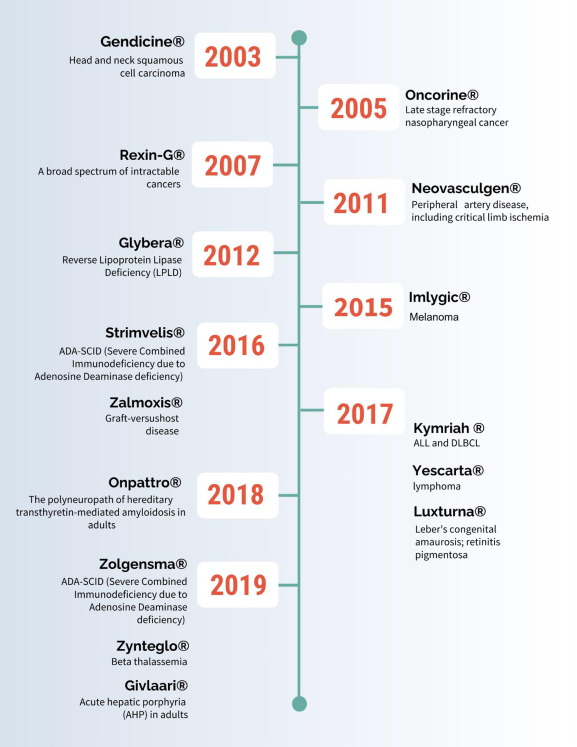
Strimvelis, In A Nutshell: Indication, Utility & Mechanism of Action
As was introduced, Strimvelis serves as a therapeutic intervention for individuals grappling with severe combined immunodeficiency due to adenosine deaminase deficiency – severe combined immunodeficiency (ADA-SCID). ADA-SCID is an uncommon genetic disorder characterized by a mutation in the gene responsible for producing the enzyme adenosine deaminase (ADA). This deficiency results in the absence of the crucial ADA enzyme, leading to compromised lymphocytes—integral white blood cells essential for combating infections. Without adequate intervention, patients with ADA-SCID typically face a grim prognosis, rarely surviving beyond two years.
Specifically designed for ADA-SCID patients lacking a suitable, matched, related donor for bone marrow transplant, Strimvelis represents a groundbreaking approach. This advanced therapy medicine falls under the category of ‘gene therapy products,’ functioning by introducing genes into the patient’s body to address the underlying genetic anomaly.
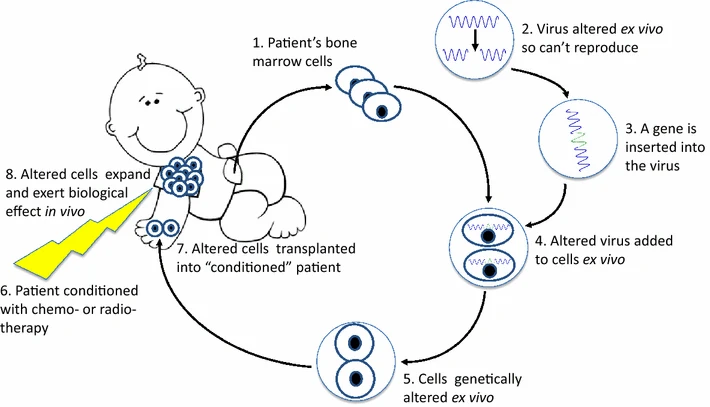
Administered exclusively under prescription, Strimvelis necessitates treatment by a seasoned physician in a specialized transplant center proficient in ADA-SCID management and the utilization of gene therapy. The preparation of Strimvelis involves collecting two bone marrow samples from the patient— one for producing Strimvelis and the other held in reserve as a precautionary measure. The infusion of Strimvelis, delivered over approximately 20 minutes through a vein, is tailored based on the patient’s body weight.
Preceding the administration of Strimvelis, patients undergo conditioning treatment with busulfan to eliminate abnormal bone marrow cells. Additionally, an antihistamine injection is administered before treatment to mitigate the risk of allergic reactions.
The intricate process of creating Strimvelis commences with the extraction of CD34+ cells— cells with the capacity to generate lymphocytes—from the patient’s bone marrow. These cells undergo genetic modification, facilitated by a retrovirus altered to carry the functional ADA gene without causing viral diseases in humans. Upon infusion into the patient’s bloodstream, Strimvelis makes its way to the bone marrow, where the modified CD34+ cells initiate the production of normal lymphocytes capable of generating ADA. The sustained presence of these improved lymphocytes enhances the patient’s immune function, alleviating symptoms associated with the compromised immune system. This transformative effect is anticipated to endure throughout the patient’s lifetime.
As Takara Bio actively promotes the supply of RetroNectin® to global clinical development initiatives, the future holds exciting prospects for Engineered Gene Therapy, marked by heightened sales growth and transformative advancements in the treatment of various genetic and rare diseases.
About TaKaRa Bio
For over five decades, Takara Bio has remained steadfast in its commitment to advancing scientific frontiers through innovative technologies. As a crucial entity within Takara Bio Inc., a global biotechnology research and development leader headquartered in Shiga, Japan, Takara Bio has played a pivotal role in shaping the landscape of life sciences. Operating from its GMP manufacturing site in West Japan, a groundbreaking facility, the company specializes in seamless and sterile handling of viruses, cells, and proteins.
The nucleus of Takara Bio’s operations lies in its headquarters situated in San Jose, CA, where diverse teams, spanning R&D, marketing, legal, finance, operations, and administration, collaborate to drive the company’s mission forward. Further supporting its functions, the main shipping and warehousing activities are efficiently managed at the Madison, WI location. Globally, Takara Bio extends its footprint with GMP stem cell facilities in Göteborg, Sweden, and the Takara Bio Europe headquarters in France, catering to regions encompassing Europe, the Middle East, and Africa.
Guided by meticulous planning, a team of dedicated researchers, and the utilization of the right tools, Takara Bio excels in providing best-in-class products, expert support, and unparalleled value to its customers. The company takes pride in its market-leading product portfolio, offering support for a diverse array of applications, ranging from CAR-T therapy, immune profiling, and genetic screening to cell and gene therapy, infectious disease research, vaccine development, drug discovery, antibiotic-resistance screening, and cancer research, among others. Takara Bio’s unwavering dedication to advancing scientific endeavors is evident in the myriad applications it supports, underscoring its pivotal role in catalyzing progress across various domains of life sciences.
Learn more about TaKaRa Bio by following this link takarabio.com.
About Fondazione Telethon ETS
Established in 1990, Fondazione Telethon ETS stands as one of the prominent biomedical charities in Italy, dedicated to propelling biomedical research aimed at the diagnosis, cure, and prevention of muscular dystrophies and various human genetic diseases. This foundation, known as Telethon Italy, exclusively concentrates its efforts on scientific research, refraining from providing healthcare services, material assistance to patients and families, or engaging in advocacy initiatives.
Over the years, Telethon Italy has played a pivotal role in advancing the field of biomedical research, channeling its mission through focused investments and support for scientific endeavors. Since its inception in 1991, the foundation has committed a substantial sum of 394 million Euros to research initiatives, fostering groundbreaking discoveries and advancements in understanding human genetic diseases.
Telethon Italy’s impact is manifested through its support for 2,477 research projects dedicated to over 445 distinct human genetic diseases. The spectrum of these projects spans from fundamental research, delving into the intricacies of genetic mechanisms, to the facilitation of clinical trials aimed at translating scientific insights into tangible medical solutions. Fondazione Telethon ETS remains steadfast in its commitment to the relentless pursuit of knowledge, aiming to unlock the mysteries surrounding genetic diseases and contribute to the broader field of biomedical science.
Check out Fondazione Telethon ETS’s Official Website to learn more.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts
Cell & Gene Therapy
Gene by Design: Mapping the Architecture and Accountability of Genetically Modified Cell Lines
A new era of quality control must accompany the rise of genome editing—one that is just as sophisticated, scalable, and transparent as the technologies that made GMCLs possible.

Cell & Gene Therapy
Eyeing the Future: Stem Cells and the Promise of Corneal Restoration
By reducing dependency on donor tissues and minimizing immunosuppressive demands, iCEPS has the potential to redefine LSCD treatment.
Read More Articles
Spatial Collapse: Pharmacologic Degradation of PDEδ to Disrupt Oncogenic KRAS Membrane Localization
PDEδ degradation disrupts KRAS membrane localization to collapse oncogenic signaling through spatial pharmacology rather than direct enzymatic inhibition.
Neumedics’ Integrated Innovation Model: Dr. Mark Nelson on Translating Drug Discovery into API Synthesis
Dr. Mark Nelson of Neumedics outlines how integrating medicinal chemistry with scalable API synthesis from the earliest design stages defines the next evolution of pharmaceutical development.
Zentalis Pharmaceuticals’ Clinical Strategy Architecture: Dr. Stalder on Data Foresight and Oncology Execution
Dr. Joseph Stalder of Zentalis Pharmaceuticals examines how predictive data integration and disciplined program governance are redefining the future of late-stage oncology development.
Exelixis Clinical Bioanalysis Leadership, Translational DMPK Craft, and the Kirkovsky Playbook
Senior Director Dr. Leo Kirkovsky brings a rare cross-modality perspective—spanning physical organic chemistry, clinical assay leadership, and ADC bioanalysis—to show how ADME mastery becomes the decision engine that turns complex drug systems into scalable oncology development programs.
Policy Ignition: How Institutional Experiments Become Durable Global Evidence for Pharmaceutical Access
Global pharmaceutical access improves when IP, payment, and real-world evidence systems are engineered as interoperable feedback loops rather than isolated reforms.