In the complex and ever-evolving landscape of pharmacology, the journey from identifying potential therapeutic agents to developing effective drugs is a blend of time-honored practices and cutting-edge innovations. One of the most critical stages in this process is lead discovery—the identification of chemical entities that exhibit the potential to treat specific biological targets or diseases. These compounds, known as lead compounds, form the foundation upon which the next generation of therapeutic agents is built. The shift from traditional drug discovery methods to more advanced lead identification approaches reflects not only a transformation in technology but also a deepening understanding of molecular biology and disease mechanisms.
The Role of Lead Compounds: Laying the Groundwork for Drug Development
At the heart of drug development lies the concept of the lead compound, a molecule that demonstrates therapeutic promise against a specific target, such as an enzyme or protein implicated in a disease. The identification of such a compound is the first critical step in drug discovery, setting the stage for further optimization. Lead compounds serve as the blueprint for developing drugs with enhanced pharmacokinetic profiles, improved safety, and superior efficacy.
The process of identifying a lead compound is not random but highly systematic, involving the screening of vast libraries of both synthetic and natural compounds. These screening efforts aim to identify molecules with selective affinity for the target of interest—whether that be a protein related to cancer cell proliferation or an enzyme involved in metabolic disorders. The initial screening helps narrow down candidates with the most promising biological activity. The lead compounds that emerge from this rigorous selection process often exhibit high selectivity, a critical factor in reducing off-target effects and increasing therapeutic potential.
While traditional drug discovery relied heavily on serendipity, where promising therapeutic molecules were often discovered by chance, modern methods have transformed this process into a data-driven, calculated endeavor. The goal now is to optimize every aspect of a compound’s interaction with its target, ensuring that only the most promising candidates progress to further stages of development.
High Throughput Screening (HTS): Speed Meets Scale
Among the modern methodologies used to identify lead compounds, High Throughput Screening (HTS) stands out for its ability to process immense volumes of data at an unprecedented pace. HTS enables the rapid testing of thousands, and sometimes millions, of compounds for their ability to interact with a biological target. Automated systems, powered by advanced robotics, efficiently assess the biological activity of these compounds, drastically reducing the time required to identify potential leads.
What makes HTS particularly valuable is its scale and speed. The ability to sift through compound libraries in such a high-volume, automated manner offers a significant advantage over earlier, more labor-intensive methods. HTS provides a broad view of the chemical landscape, allowing researchers to rapidly identify compounds with desirable biological effects. However, the method does not come without challenges. One of the major hurdles in HTS is the high incidence of false positives—compounds that may initially appear promising but lack specific activity or exhibit non-selective binding to the target.
The efficiency of HTS often comes at the cost of detailed mechanistic understanding. Although it excels at identifying compounds that interact with the target, HTS may not provide sufficient information about the precise mode of action. This limitation necessitates subsequent rounds of rigorous validation and refinement to eliminate false positives and confirm the therapeutic potential of the lead compounds. Despite these challenges, HTS remains a cornerstone of modern lead discovery, offering a powerful tool to navigate the vast and diverse chemical space.
Fragment-Based Screening: The Art of Minimalism
While HTS focuses on speed and scale, fragment-based screening takes a more meticulous and targeted approach. Instead of screening entire compounds, this method tests small, low molecular weight compounds—known as fragments—for their ability to bind to a target. These fragments are the building blocks of potential lead compounds, offering an efficient way to explore chemical space at a more granular level.
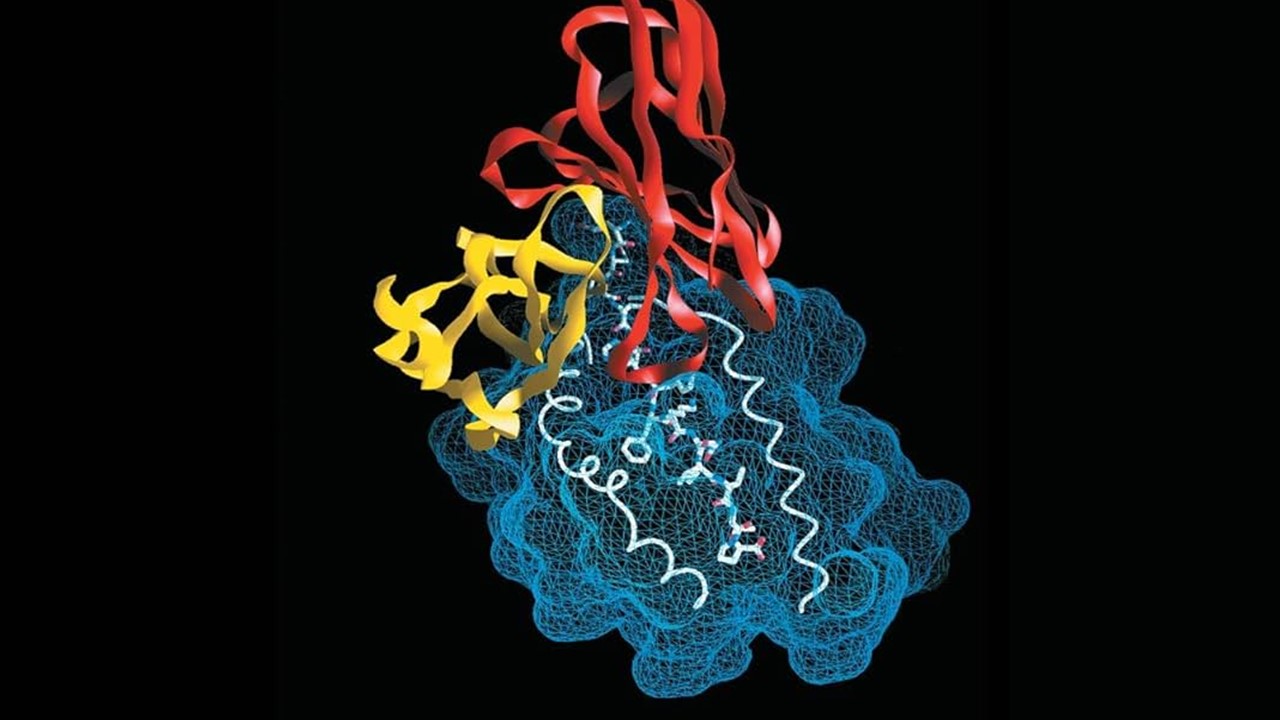
Fragment-based screening requires sophisticated structural analysis techniques such as X-ray crystallography or Nuclear Magnetic Resonance (NMR) spectroscopy to identify the precise binding sites, or “hotspots,” on the target molecule. By mapping out these key interactions, researchers can design larger, more complex molecules that build on these fragments, resulting in lead compounds with optimized binding affinities.
X-ray crystallography and Nuclear Magnetic Resonance (NMR) spectroscopy are both powerful techniques for identifying precise binding sites, or “hotspots,” on target molecules, but they operate in distinct ways. X-ray crystallography provides highly detailed, three-dimensional atomic structures by analyzing how X-rays are diffracted through a crystallized form of the molecule, offering precise spatial information about where ligands or fragments bind. However, it requires successful crystallization of the target, which can be challenging for certain proteins. NMR spectroscopy, in contrast, doesn’t require crystallization and instead uses the magnetic properties of atomic nuclei to study molecules in solution. This allows NMR to track dynamic interactions and flexibility in real time, though it is typically less detailed in resolution compared to crystallography and better suited for smaller molecules or fragments. Both techniques are instrumental in fragment-based lead discovery but offer complementary insights based on their differing strengths.
The advantage of fragment-based approaches lies in their precision. While traditional screening methods may struggle with difficult or complex targets, fragment-based screening excels by offering a deeper understanding of the target’s structure. The fragments serve as a guide for creating more potent molecules, enabling researchers to develop compounds that engage the target in a highly specific manner. This precision makes fragment-based screening particularly well-suited for addressing challenging targets, such as those involved in protein-protein interactions, which have historically been difficult to tackle with more conventional approaches.
Affinity-Based Techniques: Understanding the Strength of Interactions
In addition to HTS and fragment-based screening, affinity-based techniques offer another layer of refinement in lead discovery. These methods focus on the strength and nature of interactions between the lead compound and its target, providing insights that go beyond simple binding. Techniques such as Surface Plasmon Resonance (SPR), Isothermal Titration Calorimetry (ITC), and Bio-Layer Interferometry (BLI) are commonly employed to measure the binding kinetics, thermodynamics, and affinity of these interactions.
Surface Plasmon Resonance (SPR), Isothermal Titration Calorimetry (ITC), and Bio-Layer Interferometry (BLI) are all affinity-based techniques used to study molecular interactions, but they differ in their mechanisms and data outputs. SPR measures changes in the refractive index near a sensor surface as molecules bind, allowing real-time monitoring of binding kinetics and affinity without the need for labeling. ITC, on the other hand, directly measures the heat released or absorbed during molecular binding, providing detailed thermodynamic data like enthalpy and binding constants, but it requires larger sample quantities. BLI uses interference patterns of light reflected from two surfaces to detect binding events, offering label-free, real-time analysis with high sensitivity but requiring specific surface immobilization strategies. Each method brings unique advantages depending on the type of interaction and data needed for drug development.
Affinity-based techniques are particularly valuable in helping researchers prioritize lead compounds based on their drug-like properties. By understanding not only how strongly a compound binds to its target but also how long the interaction lasts (binding kinetics), researchers can better predict the compound’s behavior in a biological system. For example, a lead compound with optimal binding kinetics may remain attached to its target long enough to elicit a therapeutic effect, but not so long that it causes undesirable side effects. This delicate balance is critical in developing drugs that are both effective and safe.
These methods also allow researchers to probe the underlying thermodynamics of the interaction—essentially, the energy changes that occur when the lead compound binds to its target. By measuring these subtle shifts in energy, scientists gain valuable information about the stability and efficacy of the compound, enabling them to fine-tune its design.
Looking Ahead
As the field of drug discovery continues to evolve, the integration of traditional methods with modern computational tools and high-precision screening techniques has transformed lead discovery into a highly strategic and data-driven endeavor. High Throughput Screening, Fragment-Based Screening, and Affinity-Based Techniques represent just a few of the innovative approaches that have redefined how researchers identify and optimize lead compounds. These methods not only streamline the discovery process but also provide deeper insights into the molecular mechanisms that drive disease, paving the way for the development of safer, more effective drugs.
The future of lead discovery lies in the continued refinement of these methodologies, coupled with advances in computational biology, molecular modeling, and artificial intelligence. As researchers gain access to ever more sophisticated tools, the potential to discover novel therapeutics that target previously untreatable diseases grows exponentially. Ultimately, the marriage of traditional wisdom with modern innovation holds the key to unlocking the next generation of life-saving drugs.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts

Medicinal Chemistry & Pharmacology
Aerogel Pharmaceutics Reimagined: How Chitosan-Based Aerogels and Hybrid Computational Models Are Reshaping Nasal Drug Delivery Systems
Simulating with precision and formulating with insight, the future of pharmacology becomes not just predictive but programmable, one cell at a time.
Medicinal Chemistry & Pharmacology
Coprocessed for Compression: Reengineering Metformin Hydrochloride with Hydroxypropyl Cellulose via Coprecipitation for Direct Compression Enhancement
In manufacturing, minimizing granulation lines, drying tunnels, and multiple milling stages reduces equipment costs, process footprint, and energy consumption.

Medicinal Chemistry & Pharmacology
Decoding Molecular Libraries: Error-Resilient Sequencing Analysis and Multidimensional Pattern Recognition
tagFinder exemplifies the convergence of computational innovation and chemical biology, offering a robust framework to navigate the complexities of DNA-encoded science
Read More Articles
Magnetic Nanoengineering: Overcoming Biological Variability and Enhancing Therapeutic Precision
The future of nanomedicine lies in harmonizing precision, accessibility, and ecological responsibility, ushering in an era where therapies are tailored to individual biological landscapes.
Trials, Triumphs, and Trials Ahead: Navigating the Landscape of Randomized Controlled Trials in Artificial Intelligence-Driven Healthcare
The adoption of artificial intelligence in clinical practice has prompted a surge in randomized controlled trials, highlighting a balance of enthusiasm and prudence.
Illuminating the Dark Genome: Uncharted Frontiers in Therapeutic Discovery
The dark genome is not a biological void but a frontier awaiting illumination.