Navigating cultural barriers is crucial in global clinical research, where diverse perspectives often challenge the informed consent process. Community engagement emerges as a pivotal strategy, promoting ethical conduct, mutual understanding, and respect for cultural contexts.
Understanding Cultural Barriers to Informed Consent
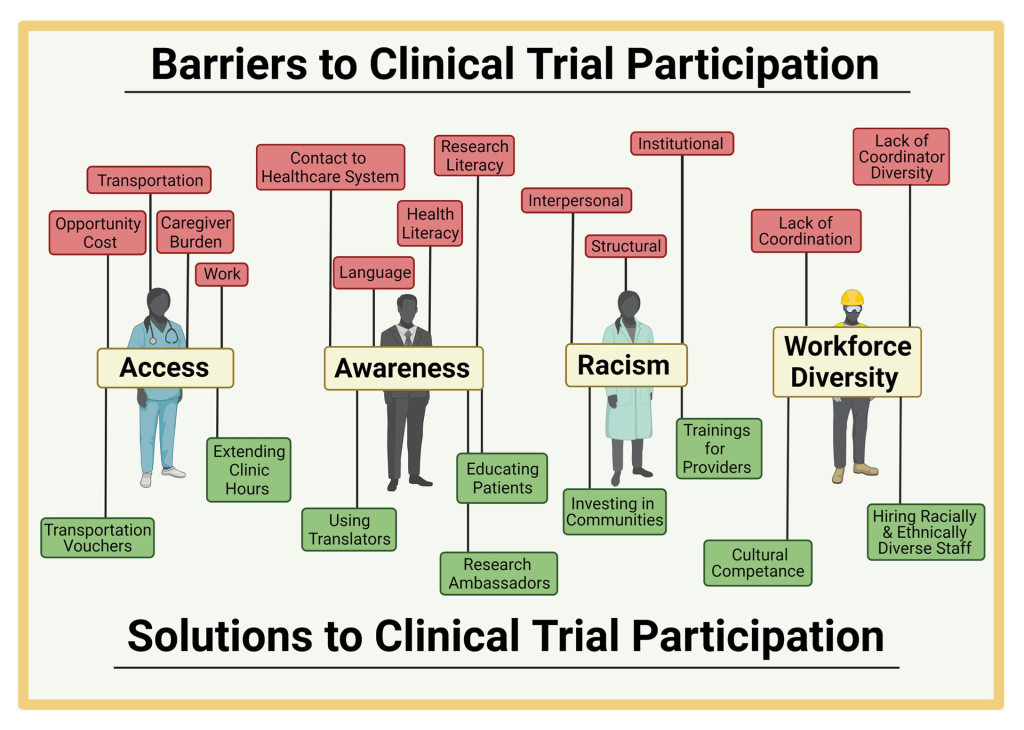
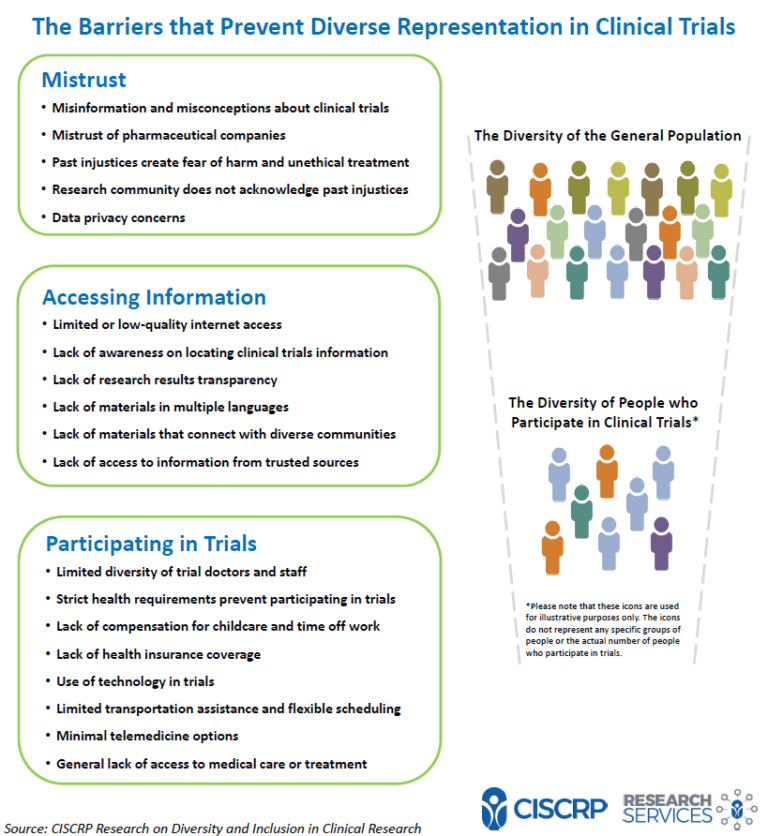
Informed consent serves as the cornerstone of ethical clinical research, facilitating communication between researchers and participants. However, in the context of global clinical trials, cultural diversity can pose significant challenges to this process. Cultural differences contribute to communication barriers, hindering participant awareness and understanding. Language disparities, lack of research awareness, and mistrust in researchers are among the key hurdles. Moreover, cultural nuances affect how health information is perceived and processed, further complicating comprehension.
Ethical Imperatives of Community Engagement
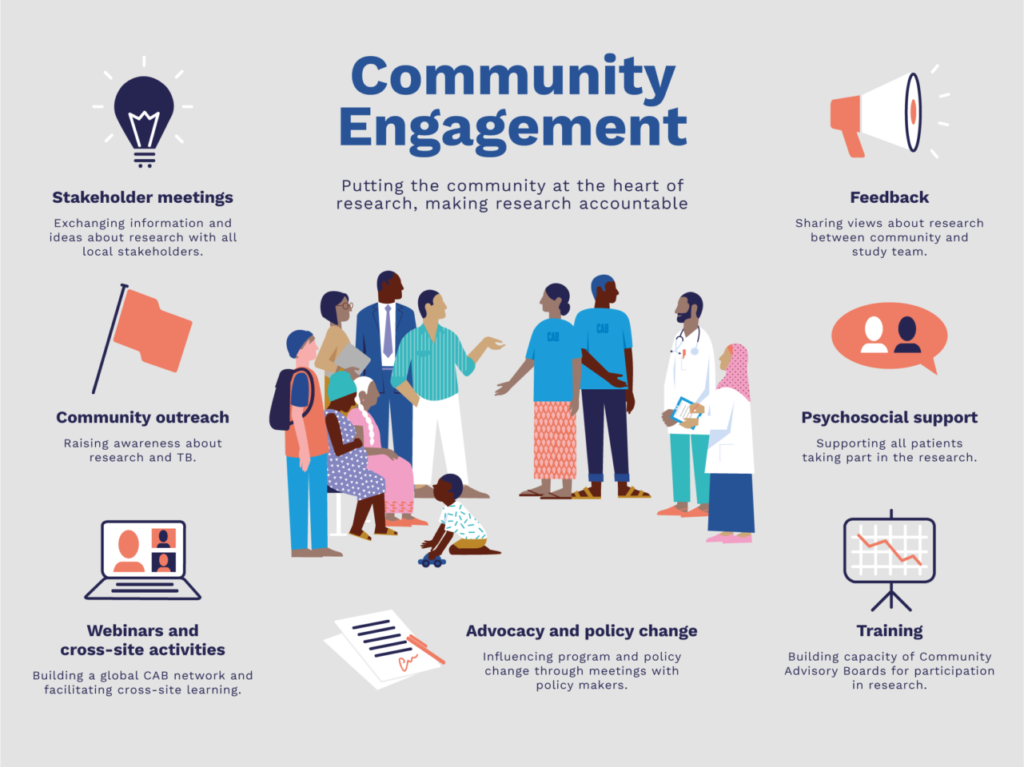
Community engagement emerges as an ethical imperative in addressing the challenges of informed consent. Recognizing communities as stakeholders in research fosters mutual accountability and ensures research relevance. It also enhances study quality by facilitating better recruitment and promoting trust-building between researchers and communities. By engaging communities, researchers demonstrate respect for local values and priorities, establishing equitable partnerships essential for ethical research conduct.
International Guidelines on Community Engagement
Global organizations like the WHO and CIOMS emphasize the significance of community engagement in research ethics. Recommendations highlight the need for a participatory approach, involving communities in research design, implementation, and dissemination. Such engagement promotes mutual learning, builds trust, and strengthens local ownership of research endeavors. It underscores the importance of respecting cultural contexts and tailoring research practices accordingly.
Strategies for Effective Community Involvement
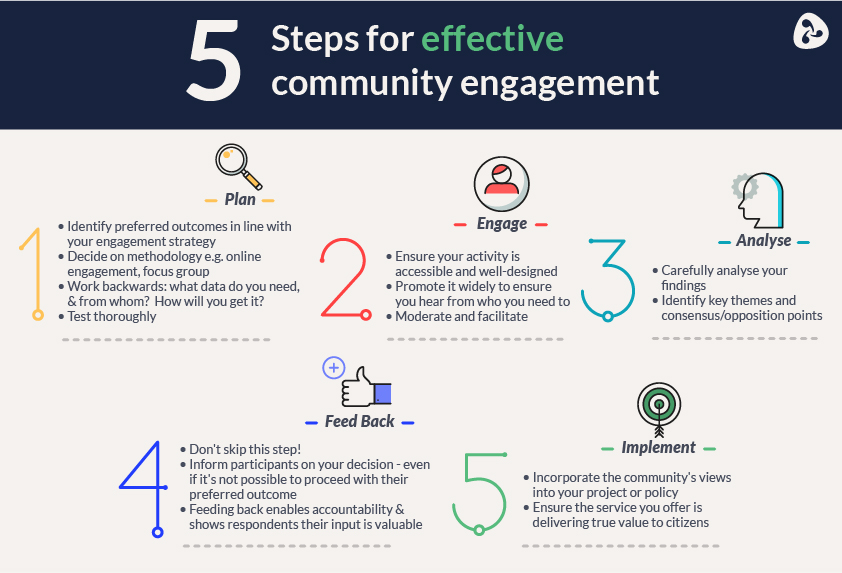
Effective community engagement requires tailored strategies aligned with local contexts. Involving community representatives in institutional review processes and fostering long-term relationships between researchers and communities are vital steps. Consultations with community members and proactive communication channels ensure ongoing dialogue and feedback loops. Leveraging local networks and embracing technological platforms also enhance outreach and engagement efforts.
Relational Autonomy: A Framework for Community Engagement
Community engagement aligns with a relational view of autonomy, recognizing individuals’ embeddedness within social networks. While upholding individual autonomy, this perspective emphasizes interdependence and communal considerations. Informed consent, viewed as a dynamic process, acknowledges the relational nature of decision-making. Community engagement practices, grounded in cultural respect and mutual trust, embody this broader ethical framework, enriching the discourse on research ethics.
In conclusion, community engagement serves as a vital bridge in overcoming cultural barriers to informed consent in global clinical research. By embracing diverse perspectives and fostering collaborative partnerships, researchers uphold ethical standards and promote meaningful participation in research endeavors.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Editor-in-Chief, PharmaFEATURES

Subscribe
to get our
LATEST NEWS
Related Posts

Clinical Trial Supply Chain
From Chaos to Control: The Clinical Reinvention of Supply Chain through Data-Driven Infrastructure
In a healthcare landscape increasingly dominated by automation and AI, it’s tempting to see technology as a cure-all.
Clinical Trial Supply Chain
Deciphering the Nexus: Cluster Analysis in Shaping Regional Supply Chain Hubs
Cluster analysis has become instrumental in understanding and optimizing supply chains.














