The timing of calorie intake synchronizes mice’s circadian cycles across several systems, according to Salk Institute researchers. The results, which were released on January 3, 2023 in Cell Metabolism, have implications for a variety of medical illnesses, including diabetes, heart disease, hypertension, and cancer, where time-restricted feeding has shown a slew of potential advantages.
TL;DR
A daily cycle of eating and fasting is part of the time-restricted feeding (TRF) behavioral nutrition intervention. TRF provides immunomodulatory health advantages in both humans and animals that come from a variety of organ systems, but it is unclear what molecules underlie these benefits. In this study, mice were given isocaloric ad libitum feeding (ALF) or TRF of a western diet, and gene expression variations in samples from 22 organs and brain regions were assessed. Samples were taken every 2 hours during a 24-hour period. The study found that TRF significantly affects gene expression. In at least one tissue, more than 80% of all genes exhibit rhythmicity or differential expression under TRF.
TRF effects on particular tissues and pathways were discovered by molecular characterization of these modifications. These research results and resources lay a solid platform for future mechanistic studies and will direct human time-restricted eating (TRE) interventions to cure a range of illness conditions, either with or without the use of pharmaceutical treatments.
Linking Nutrition with Gene Expression
Gene expression and functional changes that underlie the observed health effects are brought on by altering the type or amount of nutrients consumed. Years of research into the molecular underpinnings of these interventions point to a consistent function for the nutrition sensors mechanistic target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), sirtuins, insulin, and insulin-like growth factor-1 (IGF1) signaling in regulating responses.
Despite the fact that food and feeding interventions probably trigger responses in a variety of tissues and that the pleiotropic advantages may include inter-tissue communication, the majority of omics studies in this field of study have concentrated on a single tissue. Few studies have looked into how multi-tissue reactions to such therapies. In addition, a number of studies have demonstrated how the time of day can affect therapies like calorie restriction (CR). Understanding the worldwide ramifications of such measures is crucial, taking time of day into consideration.
Boons of Time-Restricted Eating
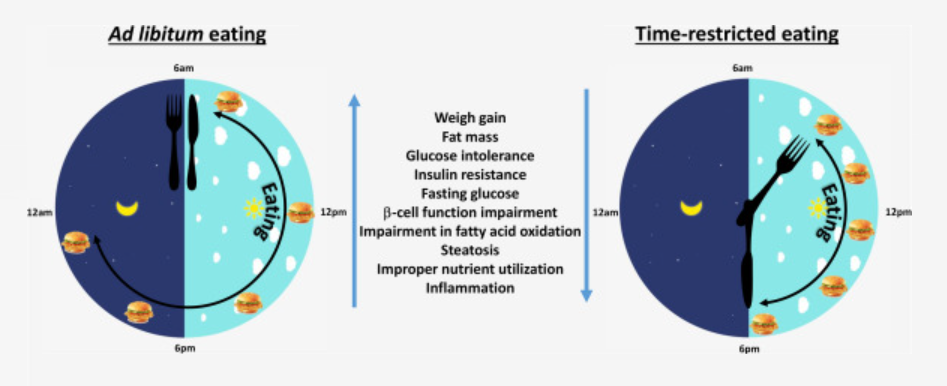
Time-restricted feeding or eating, also known as TRF or TRE in humans, is a unique strategy in which nutrients are ingested within a regular window of 8–10 hours each day, leading to pleiotropic health advantages that affect several tissues. Importantly, benefits are seen even when calorie consumption or the type of diet are left unaltered, and other human studies have also found effects that are qualitatively similar. Improvements in blood pressure, liver triglycerides, plasma lipids, heart function, gut health, exercise capacity, endurance, motor coordination, sleep, and gut health are among the advantages. There have also been decreases in tumor development, cancer risk, and the severity of neurological illnesses.
It has been demonstrated that TRF modulates diurnal fluctuations of the transcriptome (both timing and amplitude) in the Drosophila heart, mouse liver and gut, and human skeletal muscle, with matching changes in organ function, pointing toward a molecular understanding of these advantages. Other organs have not yet been investigated for transcriptome alterations, though. The term “transcriptome” describes the collection of all RNA transcripts, both coding and non-coding, found in a single cell or population of cells. It is simply the entire spectrum of messenger RNA, or mRNA, molecules that an organism expresses.
The Experimental Set-Up
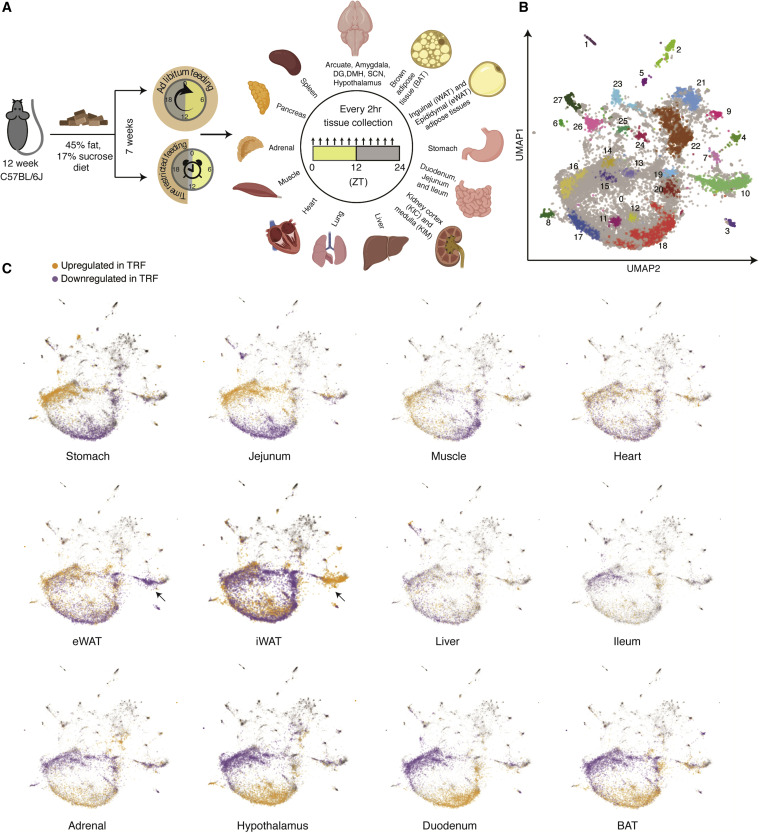
The identical high-calorie food was given to two groups of mice for the study. Free meals was made available to one group. The other group was only permitted to eat during a nine-hour timeframe each day. After seven weeks, tissue samples were taken at various times of the day and night from 22 organ groups and the brain, and they were then examined for genetic alterations. Tissues from the liver, stomach, lungs, heart, adrenal gland, hypothalamus, various kidney and intestine components, and various brain regions were among the samples.
A total of 1,035 samples that passed quality control from mice that were either given ad libitum feeding (ALF, where the diet is always accessible) or TRF were used to analyze the diurnal alterations to the transcriptome in 22 tissues. Since animals were housed in light-dark cycles, the word “diurnal” is preferred over “circadian.” Diurnal expression profiles, which are gathered under light and darkness, are thought to be more closely related to natural living conditions under the day-night cycle. Circadian expression profiles, on the other hand, are collected under light and darkness and are important for circadian mechanistic studies.
A Cornucopia of Benefits to Reap
The Rita and Richard Atkinson Chair at Salk Institute and principal author Professor Satchidananda Panda claims that the study showed a system-wide, molecular effect of time-restricted eating in mice. The findings pave the way for a closer examination of how this nutritional intervention activates genes linked to particular disorders, like cancer.
Dr. Panda and the other scientists discovered that time-restricted eating affects 70% of mouse genes. This is an intriguing discovery since it shows that altering the timing of food truly affected thousands of genes in the brain as well as the gut and liver.
Time-restricted eating also had an impact on about 40% of the genes in the brain, pancreas, and adrenal gland. The regulation of hormones depends on these organs. The body and brain need hormones to coordinate various bodily and mental processes, and hormonal imbalance has been linked to a variety of illnesses, including stress disorders and diabetes. The findings offer suggestions for how time-restricted eating can aid in the management of certain disorders.
Surprisingly, not all parts of the digestive tract were similarly affected. While time-restricted eating activated genes in the upper two regions of the small intestine, the duodenum and jejunum, the ileum, at the lower end of the small intestine, did not. This discovery could pave the way for further research into how shiftwork occupations, which disturb our 24-hour biological clock (called the circadian rhythm), affect digestive illnesses and tumors. Panda’s previous research found that time-restricted eating benefited the health of firemen, who frequently work shifts.
Furthermore, the researchers discovered that time-restricted feeding synced the circadian cycles of many organs in the body.
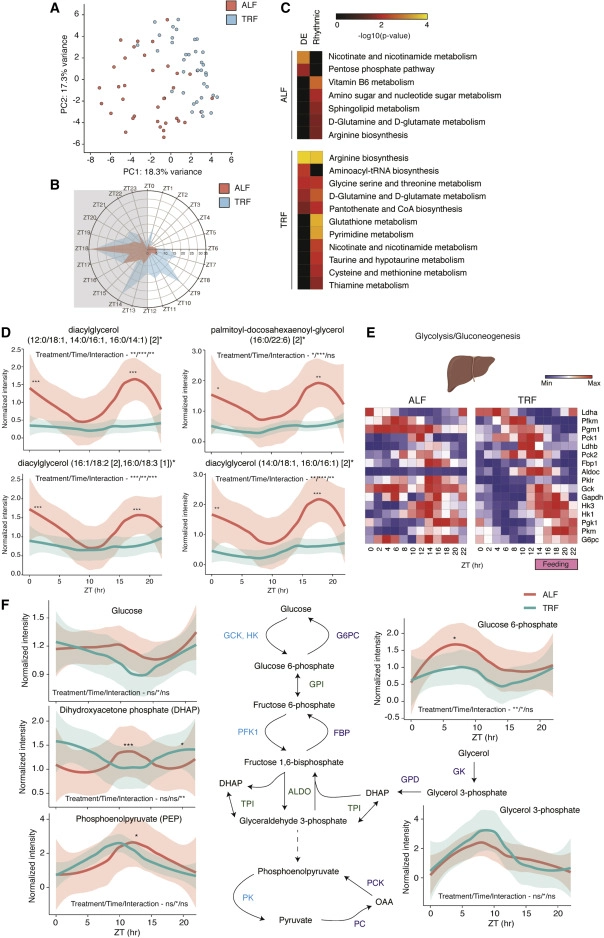
The investigation therefore demonstrated that TRF influenced the expression and/or rhythmicity of the majority of genes (80%), some in a tissue-specific manner. TRF improved the rhythmicity of gene expression across most tissues and consolidated gene expression into two discrete phases associated with fasting and feeding states. Finally, TRF promoted the rhythmic expression of genes implicated in major metabolic pathways across tissues and improved nutrition metabolism in the liver as measured by metabolite analyses.
Integrated Clockwork Orchestrates Gene Expression Rhythms
Notwithstanding the well-known pleiotropic advantages of nutritional therapies, a thorough examination of their influence on a variety of peripheral tissues and brain areas over the course of a 24-hour period has rarely been done. Here, we found that tissue-specific alterations in the relative levels and/or daily rhythms of the expression of >80% of the protein-coding genes are induced by TRF, a type of intermittent fasting. Even within the same functional class, changes in direction (up or down) may vary depending on the tissue or route.
The clock genes were rhythmic under both ALF and TRF circumstances, but in most tissues, the rhythmicity of gene expression was substantially higher under TRF (the only exceptions were SCN or suprachiasmatic nucleus and spleen). Therefore, it is hypothesized that systemic signals produced by feeding-fasting cycles in conjunction with endogenous clocks (clock modulated) may play the key role in regulating gene expression rhythmicity in peripheral organs rather than the circadian clock alone, which is the case for most tissues (clock dependent). Only 20% of the rhythmic genes in the liver were clock dependent, according to a prior study using Bmal1 or Cry1/2 mutant mice fed on normal chow during night-restricted feeding (NRF). According to the available research, this effect might also apply to peripheral organs while following a western diet.
Maximizing TRF Potential Benefits
Recent research suggests that the length and timing (day vs. night) of fasting, rather than the caloric intake or nutrient makeup, may have a significant impact on the health benefits. A number of tissue-specific transcription factors are regulated by the timing of feeding-fasting cycles, and these factors work in concert with clock proteins to induce rhythmic gene expression. Such feeding-fasting cycles do not occur during an HFD’s ALF since mice eat all day and all night long. The TRF intervention, in contrast, was found to consolidate gene expression into fasting and feeding phase peaks across all peripheral tissues. This might encourage metabolic flexibility and result in the separation of catabolic and anabolic processes.
A diet that is heavy in fat and carbs is linked to negative health effects, such as a shorter life expectancy. It’s interesting to note that TRF reversed multiple signs of aging, reducing inflammation, boosting autophagy, enhancing RNA and protein homeostasis, and increasing metabolic flux. In addition, several intervention studies including longer fasting intervals across a variety of model species and a recent publication demonstrating that autophagy promotes intermittent TRF (iTRF)-dependent expansions of longevity and health benefits in flies support this.
As a result, the gene expression profile we have identified for TRF will be a valuable tool for elucidating how TRF affects pre-clinical animal models of chronic metabolic disorders, neurodegenerative diseases, and cancer. This will support ongoing and upcoming clinical trials assessing the effectiveness of TRF in the prevention and treatment of chronic diseases.
Subscribe
to get our
LATEST NEWS
Related Posts

Sleep, Nutrition & Exercise
The Microalgae Revolution: Unlocking Sustainable Nutrition and Health Solutions
Microalgae represent a transformative solution to the challenges of feeding a growing population sustainably.

Sleep, Nutrition & Exercise
From Inactivity to Movement: The Need for Scalable Public Health Interventions
Parkrun has emerged as a potential answer to a global public health burden – physical inactivity.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.