Molecular Interactions: The Basis of Biological Complexity
Life, in its essence, hinges on the intricate interactions between four primary macromolecules: carbohydrates, lipids, proteins, and nucleic acids. Individually, these macromolecules cannot sustain life. It is their dynamic and continuous interactions, coupled with smaller organic and inorganic molecules, that generate the complexity required for life. This phenomenon, termed molecular recognition, involves biological macromolecules engaging in noncovalent interactions to form functional complexes.
Unlike a single physiological process, molecular recognition is integral to numerous biological functions, including cell signaling, genetic regulation, metabolism, and immunity. These functions rely on the precise interaction between macromolecules or between a macromolecule and a ligand. For instance, gene expression involves molecular recognition between inducing proteins and DNA, resulting in the production of mRNA. This is followed by recognition events between mRNA and ribosomes, leading to the synthesis of functional proteins.
Advancements in Molecular Recognition Models
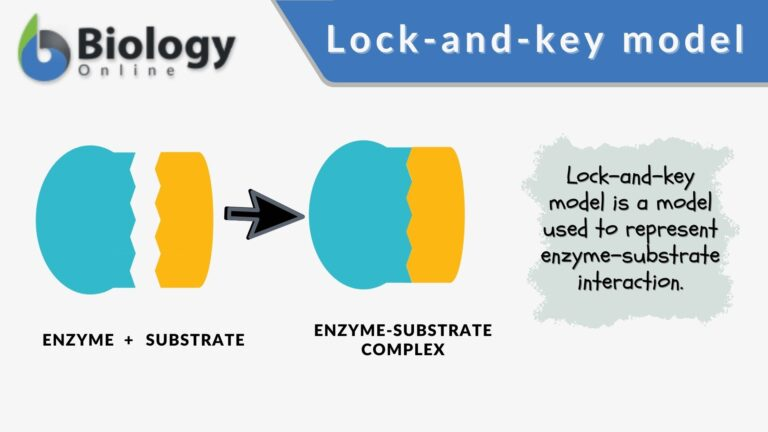
The understanding of molecular recognition has evolved significantly over the past century. In 1894, Emil Fischer introduced the “lock and key” model, suggesting that ligands and proteins maintain a rigid, highly specific interaction. This model, however, proved too simplistic as our understanding of protein dynamics grew.
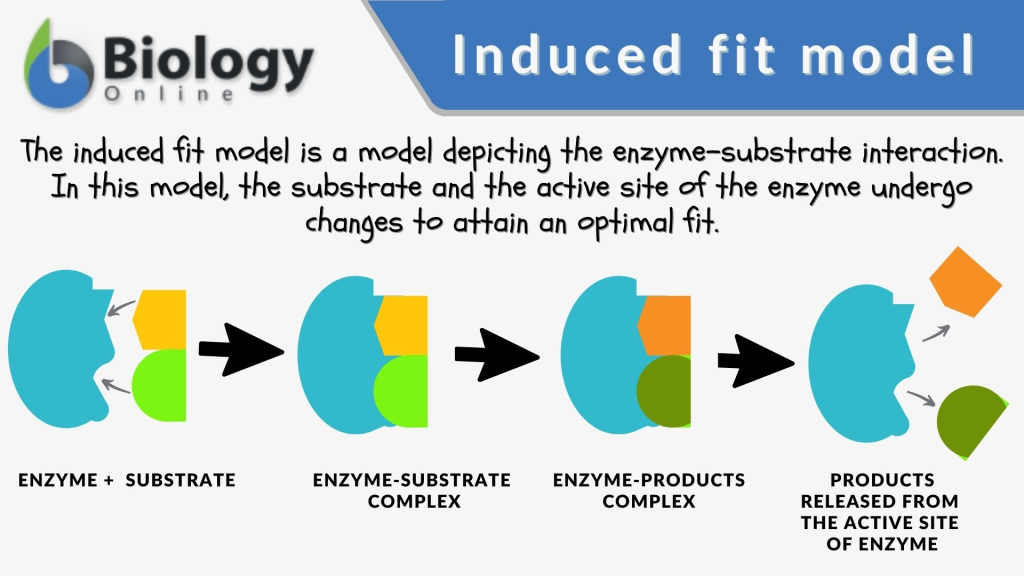
In 1958, Koshland proposed the “induced fit” model, which revolutionized the field by suggesting that proteins are not static but can undergo conformational changes upon ligand binding. This model accounted for phenomena like noncompetitive and allosteric inhibition, where proteins adapt their shape to accommodate different ligands.
More recently, the concept of conformational selection has been introduced. This model posits that proteins naturally exist in various conformations, with ligands showing different affinities for each conformation. Current understanding recognizes that induced fit and conformational selection occur in a complementary manner, providing a more comprehensive view of molecular interactions.
Molecular Recognition: A Cornerstone of Drug Discovery
The study of molecular recognition is pivotal in drug discovery. When a protein involved in a physiological process or disease is identified, it becomes a target for therapeutic intervention. Drug discovery efforts focus on identifying molecules that can interact with these target proteins to produce a therapeutic effect.
High-throughput screening (HTS) is a widely used technique to identify new molecules with potential biological activity. This automated process screens large libraries of ligands against a single protein to find those with the desired activity. Despite its efficiency, the sheer number of existing compounds makes HTS an expensive and resource-intensive initial screening method.
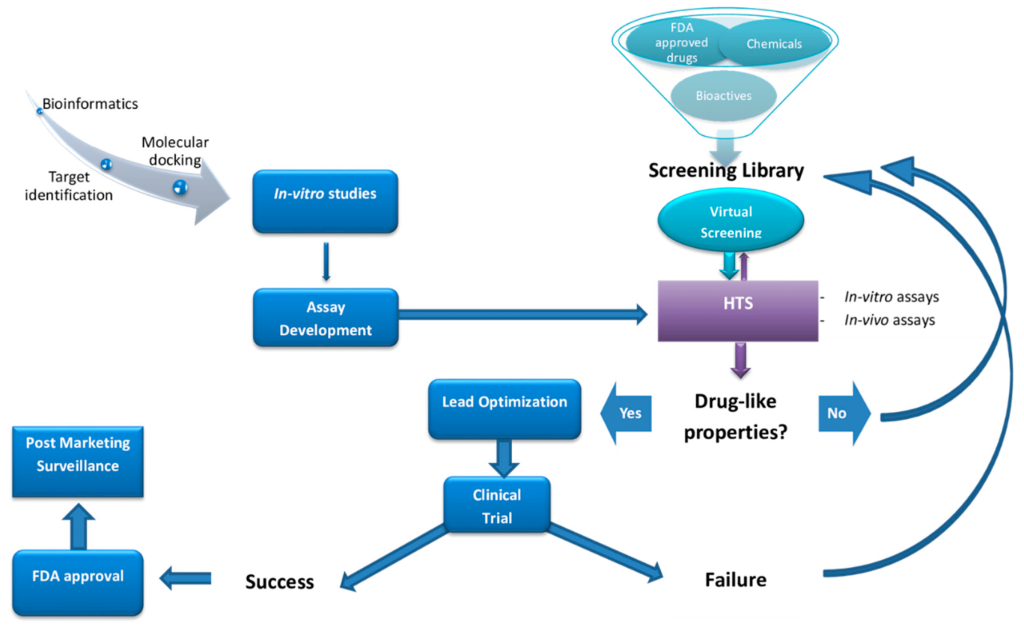
To overcome these limitations, computational methods have become essential. Structure-based virtual screening allows researchers to evaluate millions of compounds based on their affinity for the target protein. This method requires the three-dimensional structure of the target protein to perform interaction tests, known as molecular docking. Computational screening thus offers a cost-effective way to identify promising drug candidates from vast compound libraries.
The Future of Molecular Recognition
The continuous exploration of molecular recognition not only deepens our understanding of biological processes but also drives innovation in drug discovery. As models of molecular interaction become more sophisticated, they reveal the intricate and dynamic nature of macromolecular interactions. Integrating computational tools with experimental techniques holds great promise for identifying new therapeutic agents, ultimately advancing human health and well-being. The future of molecular recognition is bright, poised to unlock further secrets of life’s complex molecular dance.
Engr. Dex Marco Tiu Guibelondo, B.Sc. Pharm, R.Ph., B.Sc. CpE
Subscribe
to get our
LATEST NEWS
Related Posts

Drug Discovery Biology
Proteolytic Rewriting: Engineering Controlled Absence of Pathogenic Protein Persistence
Targeted protein degradation transforms drug therapy by engineering the cellular machinery to erase, rather than merely inhibit, pathogenic proteins.

Drug Discovery Biology
Open Nucleotides: Grant-Driven Infrastructure for Equitable mRNA Vaccine Manufacturing
By developing accessible cap analogs and RNA raw materials, Hongene Biotech, guided by David Butler’s expertise in nucleotide chemistry and supported by the Gates Foundation, is reshaping the molecular infrastructure that underpins global mRNA vaccine equity.
Drug Discovery Biology
Redox Messengers: How Endogenous Gasotransmitters Rewire Vascular Biology to Resist Age-Driven Oxidative Stress
Gasotransmitters provide a biologically sophisticated means of counteracting age-related oxidative stress and preserving vascular resilience.
Drug Discovery Biology
Aptamer Signal Dynamics: Engineering Nucleic Acid Recognition Systems for High-Fidelity, Multi-Modal Biosensing
Aptamers redefine biosensing by pairing programmable molecular recognition with versatile transduction strategies capable of detecting clinically relevant biomarkers with exceptional fidelity.
Read More Articles
Spatial Collapse: Pharmacologic Degradation of PDEδ to Disrupt Oncogenic KRAS Membrane Localization
PDEδ degradation disrupts KRAS membrane localization to collapse oncogenic signaling through spatial pharmacology rather than direct enzymatic inhibition.
Neumedics’ Integrated Innovation Model: Dr. Mark Nelson on Translating Drug Discovery into API Synthesis
Dr. Mark Nelson of Neumedics outlines how integrating medicinal chemistry with scalable API synthesis from the earliest design stages defines the next evolution of pharmaceutical development.
Zentalis Pharmaceuticals’ Clinical Strategy Architecture: Dr. Stalder on Data Foresight and Oncology Execution
Dr. Joseph Stalder of Zentalis Pharmaceuticals examines how predictive data integration and disciplined program governance are redefining the future of late-stage oncology development.
Exelixis Clinical Bioanalysis Leadership, Translational DMPK Craft, and the Kirkovsky Playbook
Senior Director Dr. Leo Kirkovsky brings a rare cross-modality perspective—spanning physical organic chemistry, clinical assay leadership, and ADC bioanalysis—to show how ADME mastery becomes the decision engine that turns complex drug systems into scalable oncology development programs.
Policy Ignition: How Institutional Experiments Become Durable Global Evidence for Pharmaceutical Access
Global pharmaceutical access improves when IP, payment, and real-world evidence systems are engineered as interoperable feedback loops rather than isolated reforms.
Sepsis Shadow: Machine-Learning Risk Mapping for Stroke Patients with Bloodstream Infection
Regularized models like LASSO can identify an interpretable risk signature for stroke patients with bloodstream infection, enabling targeted, physiology-aligned clinical management.







