Have you ever wondered how vaccines work? Why some people mount a better immune response than others? The answer lies in the complex interplay between the immune cells and molecules. Among the many players, T follicular helper (Tfh) cells are essential for orchestrating the germinal center (GC) reaction, a process critical for the generation of long-lasting immunity. However, not all Tfh cells are created equal. In a recent study published in Science Immunology, researchers have uncovered a new subset of Tfh cells that reside in the GC and possess unique regulatory functions. In this blog post, we will dive into the fascinating world of Tfh cells and their role in shaping the immune response.
The Germinal Center Reaction
The GC is a specialized microenvironment that forms in secondary lymphoid organs, such as the lymph nodes and spleen, in response to an infection or vaccination. Inside the GC, B cells undergo a process called somatic hypermutation, where their antibody genes undergo random mutations. This process generates a diverse repertoire of antibodies, some of which have higher affinity for the pathogen. The B cells that produce these high-affinity antibodies are then selected to differentiate into memory B cells or plasma cells, which produce and secrete antibodies, respectively. The GC reaction is essential for the generation of long-term protective immunity against pathogens.
The Role of T Follicular Helper Cells
Tfh cells are a specialized subset of CD4+ T cells that migrate to the GC and provide help to B cells. Tfh cells interact with B cells via the CD40-CD40L and ICOS-ICOSL pathways, which provide essential signals for B cell activation and differentiation. In addition, Tfh cells secrete cytokines, such as interleukin-21 (IL-21), which promote B cell survival and proliferation. Tfh cells are essential for the GC reaction, as their absence or dysfunction leads to impaired antibody responses and compromised immunity.
The Discovery of GC-Resident Regulatory Tfh Clones
In the recent study published in Science Immunology, the researchers sought to investigate the diversity and function of Tfh cells in the GC. Using a combination of flow cytometry, single-cell RNA sequencing, and in vitro functional assays, the researchers identified a subset of Tfh cells that expressed high levels of the transcription factor Foxp3, a hallmark of regulatory T cells. These Tfh clones, which the researchers termed “regulatory Tfh,” or “Tfr” cells, were found to suppress B cell responses in vitro, suggesting that they have a negative regulatory role in the GC reaction. Furthermore, the researchers found that Tfr cells were clonally expanded, indicating that they have undergone antigen-driven selection, similar to B cells.
Implications for Vaccine Development and Autoimmune Diseases
The discovery of Tfr cells sheds new light on the complexity of the GC reaction and its regulation. The presence of Tfr cells suggests that the immune system has evolved mechanisms to prevent excessive or prolonged B cell responses, which can lead to autoimmunity or tissue damage. The identification of Tfr cells also opens up new avenues for vaccine development. By manipulating the Tfh/Tfr balance, researchers may be able to enhance or suppress specific B cell responses, depending on the type of vaccine or disease. Finally, the discovery of Tfr cells may have implications for autoimmune diseases, where dysregulation of Tfh/Tfr balance has been implicated in the pathogenesis of diseases such as systemic lupus erythematosus (SLE).
Conclusion
The immune system is a complex and dynamic network of cells and molecules that work together to protect us from pathogens and diseases. Tfh cells are a critical component of this system, and the discovery of Tfr cells adds a new layer of complexity to their function. The GC reaction is a delicate balance between B cell activation and regulation, and Tfh/Tfr cells play a crucial role in this process. The identification of Tfr cells may have far-reaching implications for vaccine development, autoimmune diseases, and other areas of immunology research. As we continue to unravel the mysteries of the immune system, we are one step closer to developing new therapies and interventions that can improve human health.
Study DOI: 10.1126/sciimmunol.ade8162
Subscribe
to get our
LATEST NEWS
Related Posts

Immunology & Oncology
The Silent Guardian: How GAS1 Shapes the Landscape of Metastatic Melanoma
GAS1’s discovery represents a beacon of hope in the fight against metastatic disease.
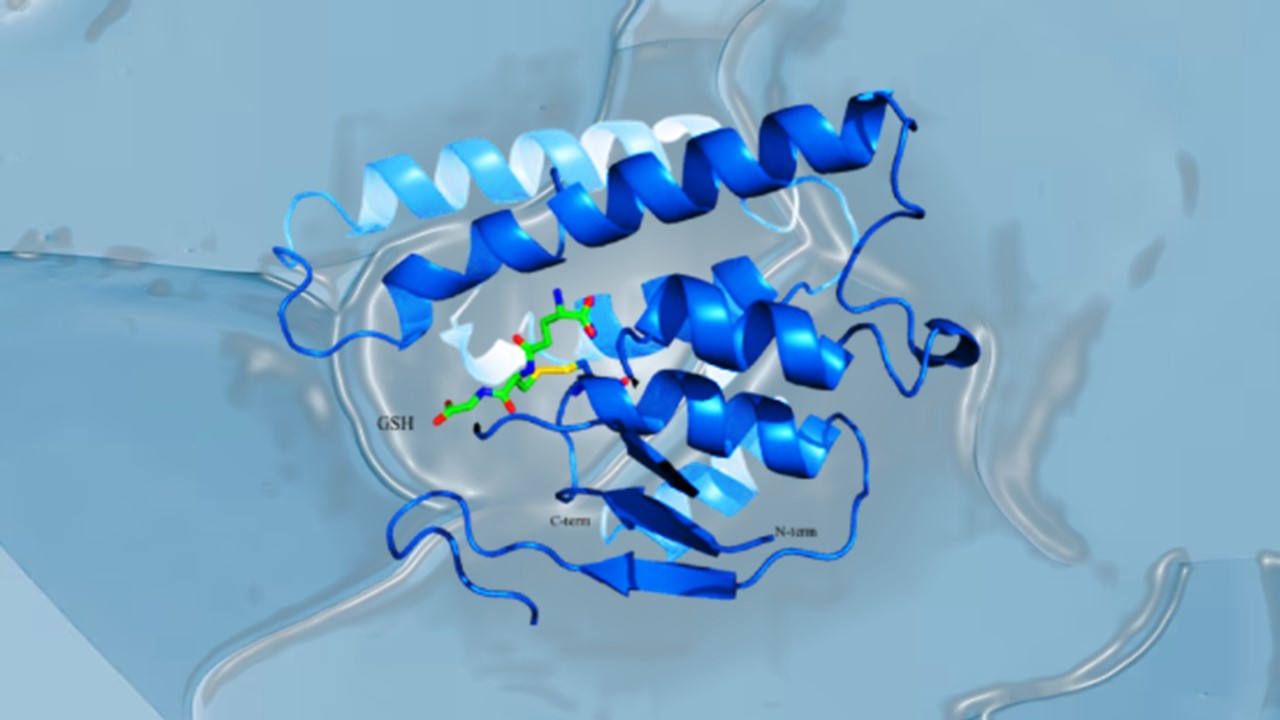
Immunology & Oncology
Resistance Mechanisms Unveiled: The Role of Glutathione S-Transferase in Cancer Therapy Failures
Understanding this dual role of GSTs as both protectors and accomplices to malignancies is central to tackling drug resistance.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.
Designing Better Sugar Stoppers: Engineering Selective α-Glucosidase Inhibitors via Fragment-Based Dynamic Chemistry
One of the most pressing challenges in anti-diabetic therapy is reducing the unpleasant and often debilitating gastrointestinal side effects that accompany α-amylase inhibition.