Let’s Drive Into the World of CAR-T
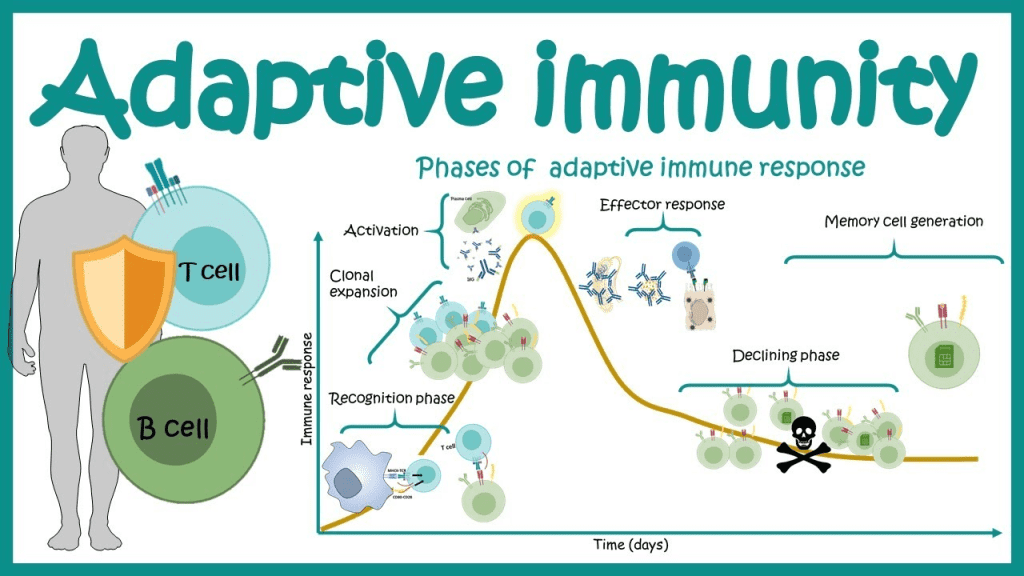
The way your immune system functions is by monitoring every component that is typically found in your body. The immune system attacks every new substance it doesn’t identify since it triggers an alarm.
Chimeric antigen receptor (CAR) T-cell therapy is a technique for getting immune cells called T cells (a kind of white blood cell) to fight cancer by altering them in the lab so they can locate and eradicate cancer cells. Due to its use of modifying T cells’ own genetic makeup to aid in their fight against cancer, CAR T-cell therapy is also occasionally referred to as a form of cell-based gene therapy.
Even when other treatments are no longer viable, this kind of treatment can be quite effective in treating some types of cancer.
CAR T cell therapy may improve the efficacy of surgery for solid tumors, according to a preclinical study conducted by scientists at the Perelman School of Medicine at the University of Pennsylvania.
How CAR-T Goes Vroom Vroom
By locating proteins known as antigens on the surface of those cells, the immune system can identify foreign substances in the body. T cells, which are immune cells, have their own proteins called receptors that connect to foreign antigens and aid in causing other immune system components to start destroying the foreign object.
Antigens and immunological receptors have a similar relationship to a lock and key. Each foreign antigen has a particular immune receptor that can attach to it, similar to how a lock can only be opened with the proper key.
Cancer cells include antigens as well, but if your immune cells lack the proper receptors, they won’t be able to bind to the antigens and aid in the eradication of the cancer cells.
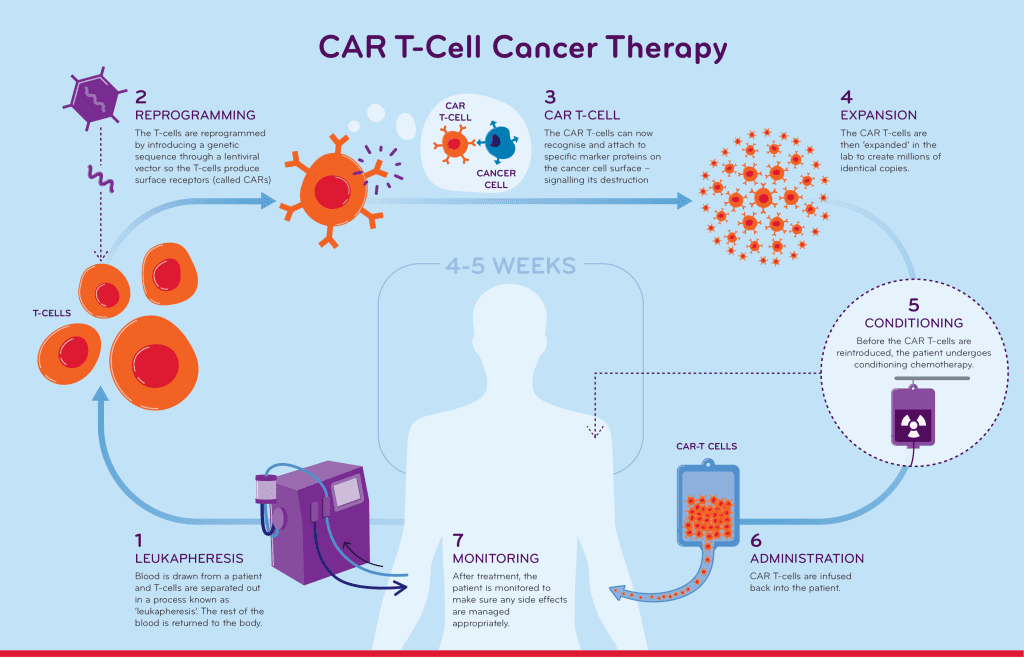
When T cells from the patient’s blood are utilized in CAR T-cell therapy, T cells are transformed in the lab by the insertion of a gene for a receptor (known as a chimeric antigen receptor, or CAR), which helps the T cells adhere to a specific cancer cell antigen. The patient is then given another round of CAR T cells.
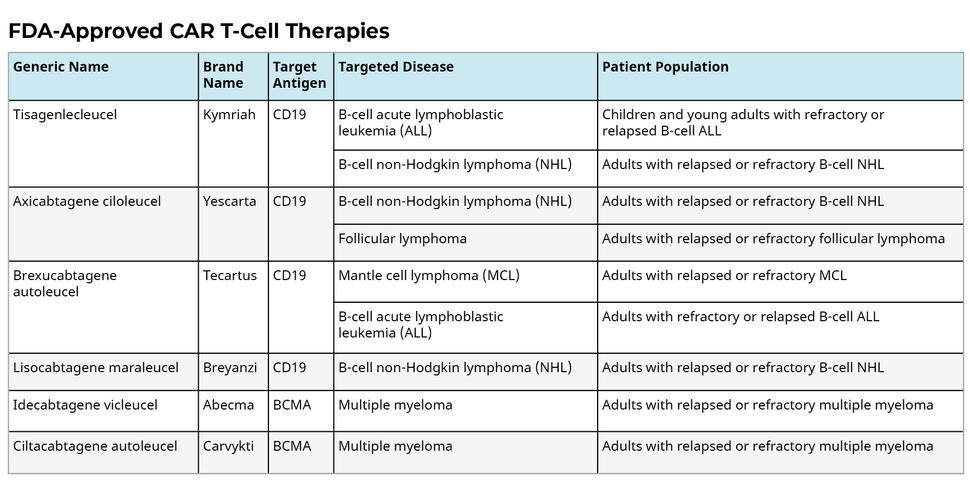
Due to the fact that different cancers have different antigens, each CAR is developed targeting a particular cancer antigen. For instance, the CD19 antigen is present on the surface of certain leukemia or lymphoma cancer cells. The CAR T-cell therapies used to treat malignant malignancies are made to bind to the CD19 antigen; they cannot treat tumors that do not express this antigen.
It Takes More Than Incisions
A unique gel containing human CAR T cells was given to surgical incisions in mice after partial tumor excision in the study, which was just released this Wednesday, January 11, 2022, in Science Advances. They discovered that almost always, the CAR T cells appeared to eradicate the remaining tumor cells, allowing the mice to survive when they would have otherwise perished from tumor recurrence.
When cancer has not spread from a solid tumor, surgery may be curative. Nonetheless, it is frequently very challenging for surgeons to determine the boundary between a tumor and healthy tissue. As a result, post-surgical recurrence from tiny tumor cells is typical for many cancer types. In order to eradicate any leftover tumor cells, one potential solution to this issue is to administer an anti-tumor treatment to the remaining tissue margins right away after the tumor has been removed. CAR T cells were used in the study by Penn researchers to test that strategy.
Finding uses for CAR T cell therapy in solid tumors is a key objective as we move the treatment’s development ahead. This is according to the study’s principal author Carl June, MD, who also serves as the center’s director and the Richard W. Vague Professor of Immunotherapy at Penn Medicine’s Abramson Cancer Center. The Penn researchers have organized a clinical trial for people with locally advanced breast cancer in light of the encouraging findings in this investigation.
Each and every CAR T therapy that has been authorized for clinical usage targets proteins identified on cancer cells. June and colleagues at Penn helped design and test what became, in 2017, the first U.S.-made T cell therapy. Usually, the T cells are extracted from the patient’s blood, modified in the lab, and then re-injected into the patient to work as a “living medication.” Treatment with CAR T cells that has received FDA approval. There are currently six CAR T cell therapies with FDA approval that target different types of blood malignancies, giving patients who have exhausted all other conventional alternatives new hope.
Same Sweeping Approach, Different Dumpsites to Clear
Due in part to tumor mass and tumor immune responses, solid tumors have so far proven to be more difficult to treat with CAR T therapies. CAR T cells may only be beneficial for the more limited purpose of eliminating cancer cells that remain after surgery, according to studies done last year by another team of scientists using a mouse model of brain cancer. The most prevalent form of pancreatic cancer, human pancreatic ductal carcinoma, was tested in the current study together with triple-negative breast cancer, which lacks all three of the primary breast cancer markers. Both of these solid tumor varieties are infamously challenging to treat.
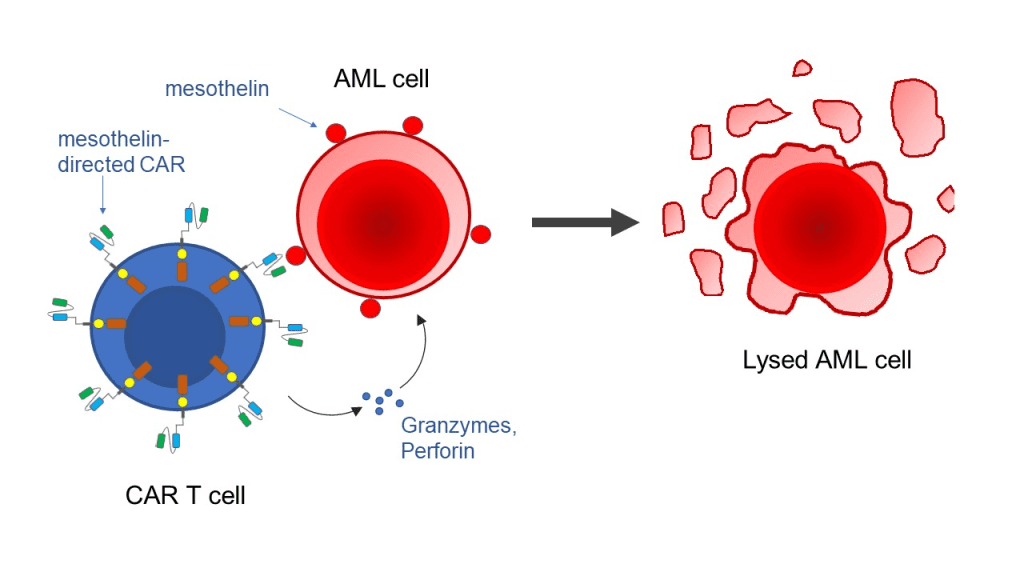
Mesothelin, a surface marker on both kinds of tumor cells used in the trials, was used to direct the CAR T cells to their target. The residual tumor tissue expanded in the absence of the CAR T cells and fibrin gel, and the animals died after around seven weeks. However, when given the gel, 19 out of 20 mice with leftover tumor tissue experienced a rapid disappearance of this tissue, and these mice continued to live normally for the rest of the observation time without experiencing any evident wound-healing issues or other negative effects.
Additional research revealed that CAR T cells that target mesothelin have the potential to attack healthy cells that contain that protein marker after intravenous injection. Additionally, the toxicity was reduced by local injection of the CAR T cells as opposed to direct injection of the cells into the blood.
The efficacy of CAR T as a supplement to surgery for solid tumors is demonstrated by this study. Along with delivering CAR T cells in conjunction to other cellular therapies and anticancer medicines, the aforementioned strategy may be expanded in the future, perhaps increasing its anticancer potency yet further.
Subscribe
to get our
LATEST NEWS
Related Posts

Immunology & Oncology
The Silent Guardian: How GAS1 Shapes the Landscape of Metastatic Melanoma
GAS1’s discovery represents a beacon of hope in the fight against metastatic disease.

Immunology & Oncology
Resistance Mechanisms Unveiled: The Role of Glutathione S-Transferase in Cancer Therapy Failures
Understanding this dual role of GSTs as both protectors and accomplices to malignancies is central to tackling drug resistance.
Read More Articles
Myosin’s Molecular Toggle: How Dimerization of the Globular Tail Domain Controls the Motor Function of Myo5a
Myo5a exists in either an inhibited, triangulated rest or an extended, motile activation, each conformation dictated by the interplay between the GTD and its surroundings.
Designing Better Sugar Stoppers: Engineering Selective α-Glucosidase Inhibitors via Fragment-Based Dynamic Chemistry
One of the most pressing challenges in anti-diabetic therapy is reducing the unpleasant and often debilitating gastrointestinal side effects that accompany α-amylase inhibition.